Legend Biotech’s BCMA CAR-T Therapy Carvykti (Ciltacabtagene Autoleucel) Package Insert
Legend Biotech’s BCMA CAR-T Therapy Carvykti (Ciltacabtagene Autoleucel) Package Insert
On February 28, 2022, the FDA officially approved Ciltacabtagene autoleucel, a BCMA CAR-T therapy developed by Legend Biotech/Janssen, for the treatment of relapsed or refractory multiple myeloma. The commercial name for this product is Carvykti.
Legend Biotech/Janssen priced Carvykti at $465,000, higher than the $419,500 price tag for Abecma, the BCMA CAR-T therapy developed by Bristol Myers Squibb.

On March 3, the FDA officially released the prescribing information for Carvykti on its website.

The dosage of Legend Biotech/Janssen’s Carvykti is 0.5-1.0×10^6/kg, with a maximum of 1×10^8 cells. In contrast, the dosage of Bristol Myers Squibb’s Abecma is 3.0-4.6×10^8 cells, which is more than three times higher than Carvykti.
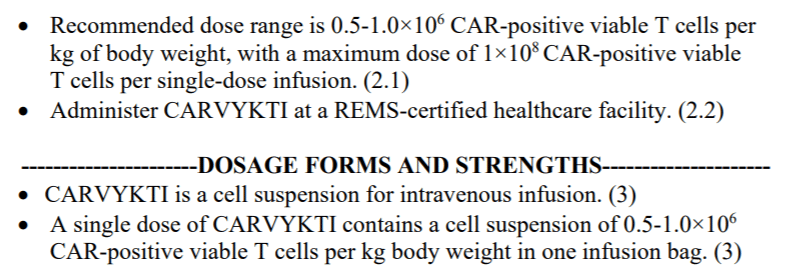
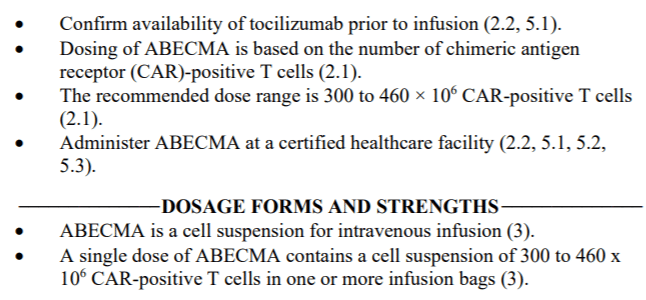
Carvykti employs a bispecific nanobody design and demonstrated an overall response rate (ORR) of 97.9% and a stringent complete response (sCR) rate of 78.4%, with a median duration of response (mDoR) of 21.8 months.
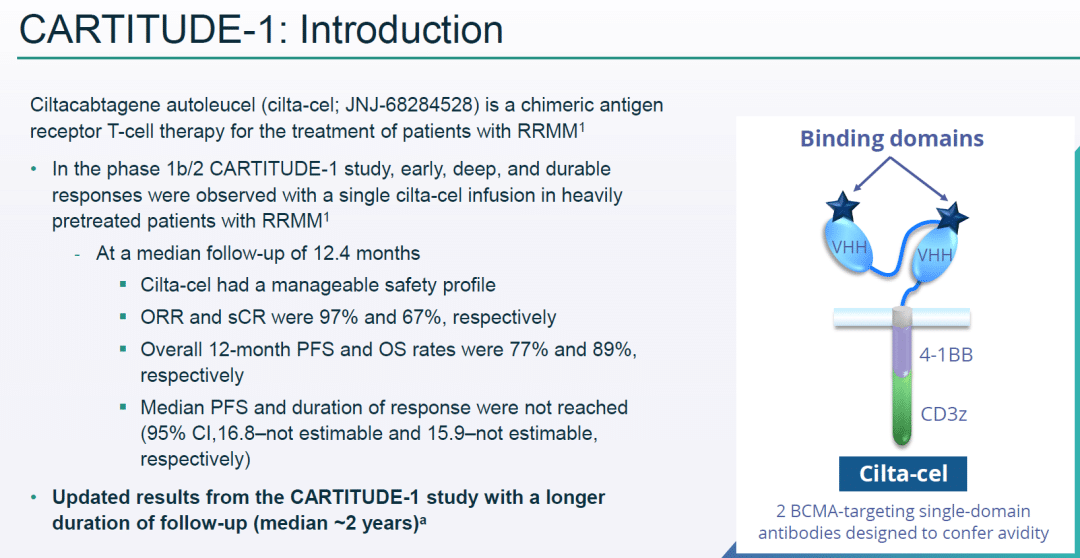
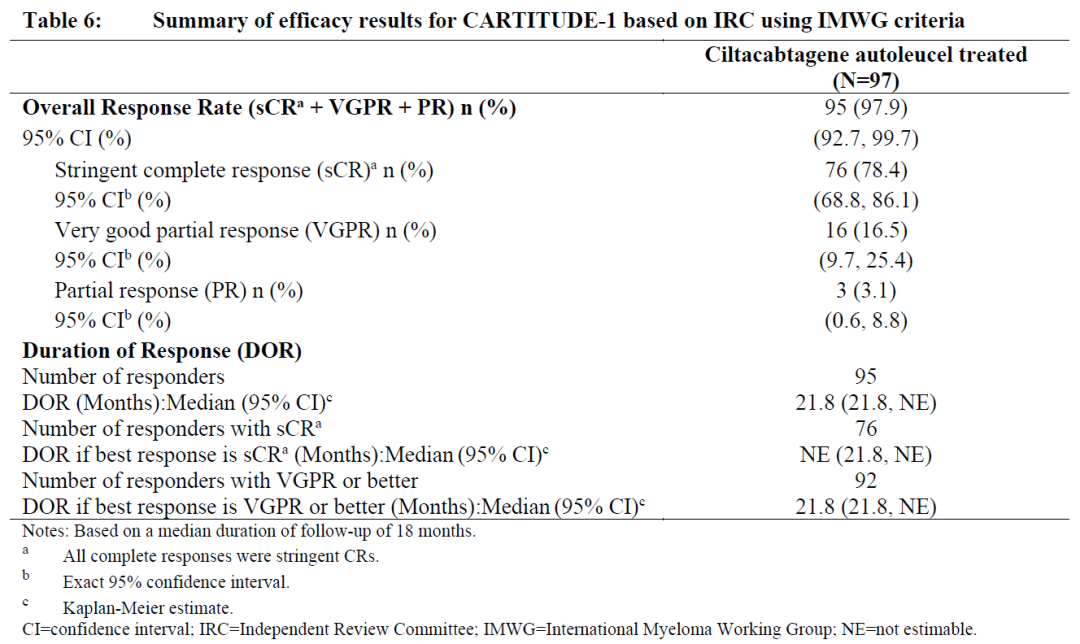
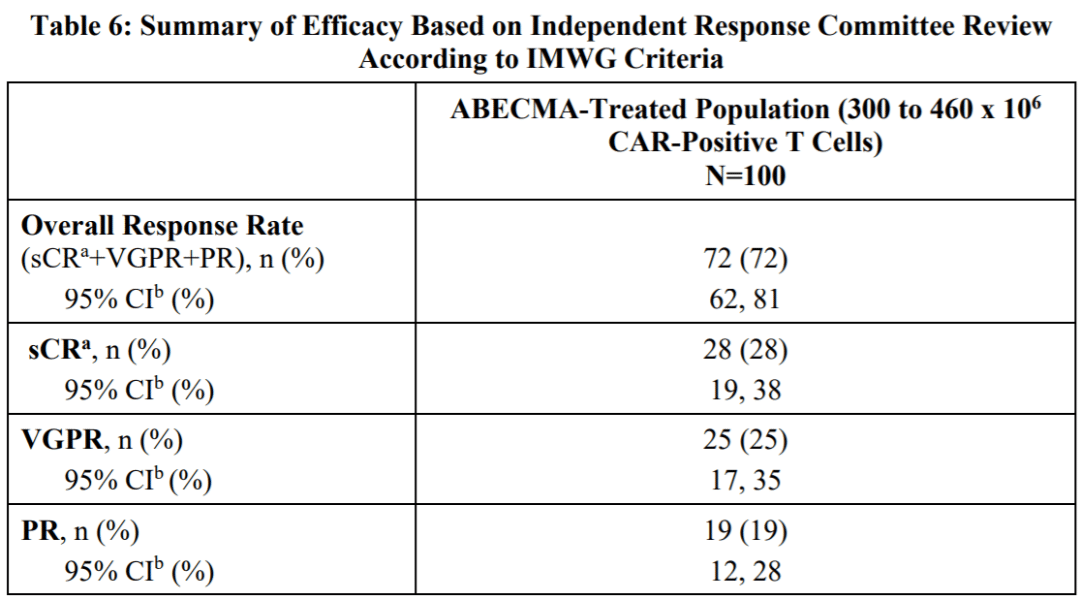
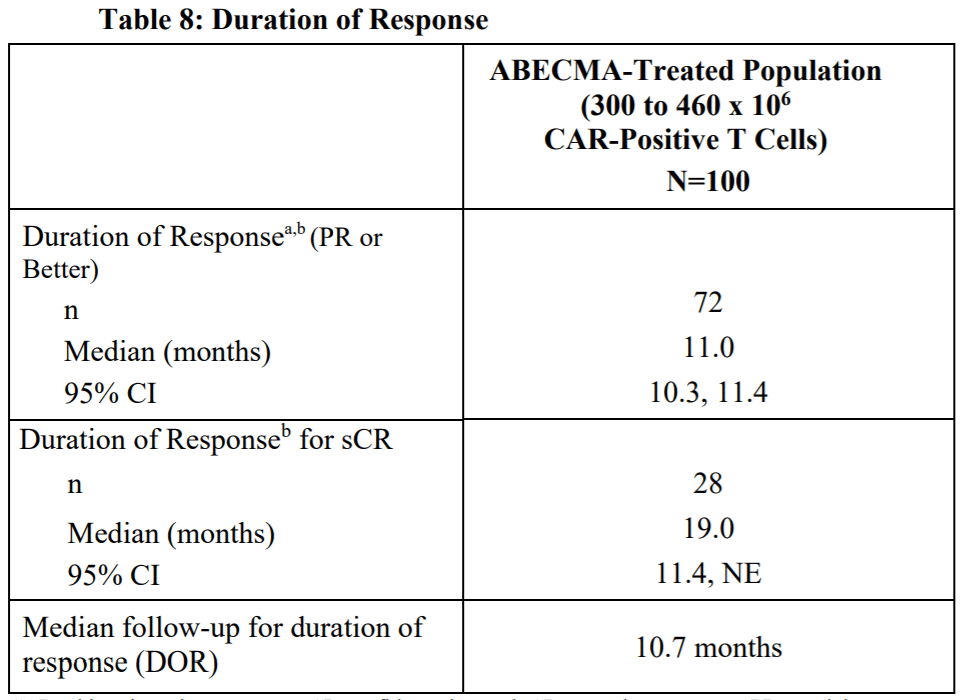
In comparison, Abecma had an ORR of 72%, sCR of 28%, and mDoR of 11 months. When compared side by side, Carvykti demonstrated superior efficacy.
Carvykti may contain a small amount of NK cells.

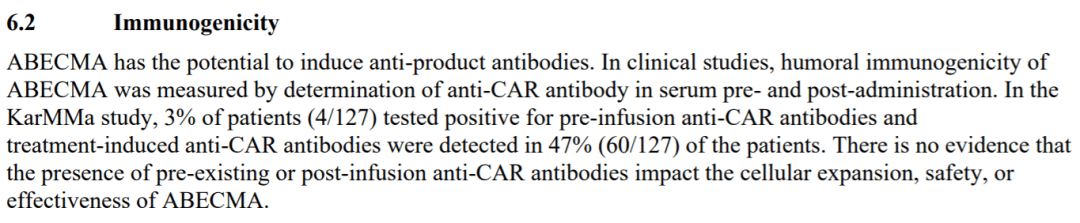
The incidence of anti-drug antibodies (ADA) was 19.6% for Carvykti and 47% for Abecma.

In summary, Legend Biotech’s successful approval of Carvykti paved the way for innovative Chinese biopharmaceuticals to enter the European and American markets. It also highlighted the clinical value and market value of differentiated innovative products. In the coming years, more and more innovative drugs developed in China will compete in the global market.
Content Source:医药笔记
