Research Progress of CAR-T Myeloma Therapy
Research Progress of CAR-T Myeloma Therapy
Multiple myeloma (MM) is a malignant plasma cell neoplasm characterized by the abnormal accumulation of monoclonal plasma cells in the bone marrow and the production of monoclonal immunoglobulins or their fragments (M-protein), leading to organ and tissue damage. Hypercalcemia, renal failure, anemia, and bone destruction are common clinical manifestations. In recent years, the global incidence of MM has increased significantly, by 126% from 1990 to 2016, making it the second most common malignant hematological malignancy. Although significant progress has been made in the treatment of multiple myeloma with proteasome inhibitors, immunomodulators, and monoclonal antibodies, relapse and drug resistance remain major challenges, and MM is currently incurable. Therefore, the progress of CAR-T therapy for multiple myeloma is of great significance.
1、CAR-T Therapy
Chimeric antigen receptor T-cell (CAR-T) immunotherapy is a novel therapeutic approach that utilizes the patient’s own immune system to combat cancer cells. Simply put, it involves isolating T cells from a patient’s peripheral blood, genetically modifying these cells to express CAR through gene transfer technology, and then reinfusing them into the patient for anti-tumor treatment.
A CAR consists of four components: a single-chain variable fragment (scFv) derived from a monoclonal antibody, a hinge region from CD8, a transmembrane domain, and intracellular signaling domains (Figure 1).
The first-generation CAR contained only one intracellular signaling domain; the second generation incorporated costimulatory domains such as CD28 or 4-1BB to enhance the anti-tumor effect of CAR-T cells; the third generation further increased the number of costimulatory domains to two based on the second generation to promote CAR-T cell proliferation and activation; the fourth generation added cytokines to the third generation to enhance the cytotoxicity of CAR-T cells against tumors.
Researchers have developed a novel CAR construct, a new generation of CD19 CAR encoding a truncated cytoplasmic domain from the interleukin (IL)-2 receptor β chain (IL-2Rβ) and a STAT3-binding tyrosine-X-X-glutamine (YXXQ) motif, as well as T-cell receptor (TCR) signaling (CD3ζ) and costimulatory (CD28) domains. This is considered the fifth generation of CAR, although this concept is currently exploratory. Compared to CAR-T cells expressing CD28 or 4-1BB costimulatory domains alone, this construct exhibited superior in vivo persistence and anti-tumor activity in hematological and solid tumor models, offering hope for the treatment of solid tumors.
After CAR-T cell infusion, the engineered T cells can specifically recognize and kill tumor cells expressing the target antigen through three mechanisms: cell lysis, cytokine release, and Fas/FasL, independent of major histocompatibility complex (MHC) restriction.
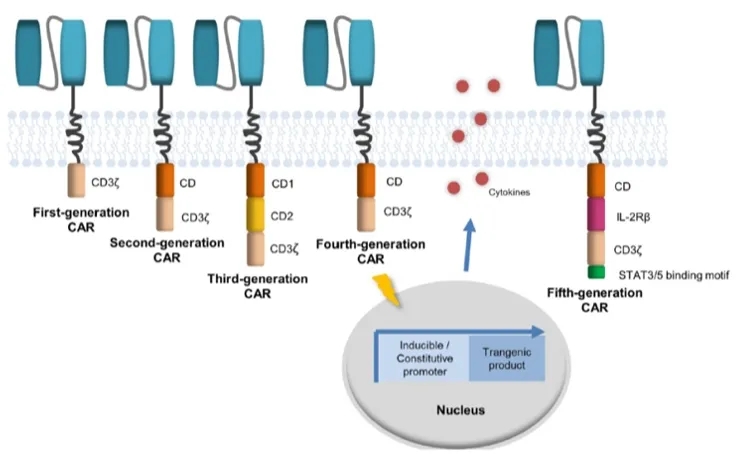
Figure 1: Schematic diagram of CAR structure
2、Targets for MM CAR-T Therapy
Currently, B-cell maturation antigen (BCMA), signaling lymphocyte activation molecule family member F7 (SLAMF7), and G protein-coupled receptor class C group 5 member D (GPRC5D) have been used as targets for MM CAR-T therapy.
Three BCMA-targeted CAR-T products have been approved for the market, including bb2121, which was approved in March 2021, cilta-cel approved in February 2022, and the most recently approved Equecabtagene Autoleucel, co-developed by Chinese companies IASO Bio and Innovent Biologics. Studies on other antigens are also underway.
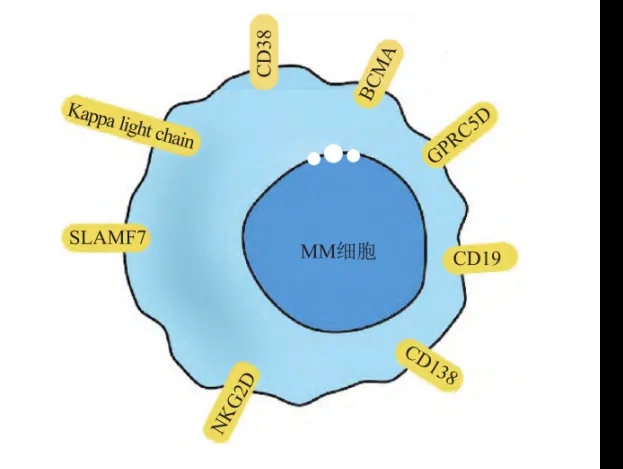
Figure 2: Therapeutic targets for MM
1.BCMA
BCMA is a type III transmembrane protein belonging to the tumor necrosis factor receptor (TNFR) family. It is upregulated in the late stages of B-cell maturation, selectively expressed on plasma cells, and highly expressed on most multiple myeloma cells, but not on immature B cells, memory B cells, or other normal tissue cells. BCMA binds to B-cell activating factor (BAFF) to activate the NF-κB and MAPK/JNK pathways, regulating the survival of long-lived plasma cells, and can also promote the proliferation of MM cells in the bone marrow microenvironment by binding to proliferation-inducing ligand (APRIL).
bb2121, developed by Bluebird Bio, is a CAR-T product transduced with a lentiviral vector encoding a BCMA-specific CAR. Raje et al. reported a study of bb2121 for the treatment of relapsed/refractory multiple myeloma. A total of 36 patients with relapsed/refractory MM were enrolled, with 3 patients dropping out of the study due to disease progression prior to infusion. The overall response rate for the remaining 33 patients was 85%, with 45% of patients achieving complete response (9%) or stringent complete response (36%).
Additionally, cilta-cel, produced by Johnson & Johnson, is another BCMA-targeted CAR-T product. Unlike other anti-BCMA CAR-T therapies, it directly targets two BCMA epitopes (VH1 and VH2), improving its affinity for BCMA-expressing cells. Lin et al. reported early, deep, and durable responses in patients treated with cilta-cel. A total of 97 patients were included in the study and received a single infusion of 0.75 × 10^6/kg cilta-cel cells after lymphodepletion on days 5-7. The overall response rate (ORR) was 97.9% (95% CI: 92.7-99.7), with 94.9% of patients achieving very good partial response (VGPR) and 82.5% achieving stringent complete response (sCR). Among the 61 patients evaluable for minimal residual disease (MRD), 92% were MRD-negative, with 44% (27/61) maintaining MRD negativity for ≥6 months. The 2-year progression-free survival (PFS) rate for patients with sustained MRD negativity ≥6 months was 91%, and 18% of patients remained MRD-negative for >12 months. While anti-BCMA CAR-T therapy has achieved satisfactory clinical efficacy in MM treatment, and patients with relapsed/refractory MM can also significantly benefit from anti-BCMA CAR-T therapy, these products have also been associated with a high risk of cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS). Improving the safety and efficacy of these products remains a challenge to be addressed.
2.SLAMF7
SLAMF7, also known as CD319, CRACC, or CS1, is a member of the signaling lymphocyte activation molecule (SLAM) family and plays a crucial role in regulating immune cell function. In normal tissues, SLAMF7 expression is limited to the hematopoietic system, including NK cells, some T cells and B cells, monocytes, macrophages, and dendritic cells. During the normal B-cell life cycle, SLAMF7 is highly expressed on pre-B cells and plasma cells and is also highly expressed on malignant plasma cells in multiple myeloma, monoclonal gammopathy of undetermined significance (MGUS), and smoldering myeloma. Studies have shown that SLAMF7 is uniformly expressed on malignant plasma cells in newly diagnosed (ND) myeloma and is retained after intensive chemoradiotherapy in relapsed myeloma, with no known expression in other normal human tissues. This finding has made SLAMF7 a potential target for CAR-T cell therapy in myeloma.
3.GPRC5D
G Protein-Coupled Receptor Family C Group 5 Member D (GPRC5D) is a transmembrane receptor protein primarily expressed on the surface of plasma cells. Compared to normal cells, the expression level of GPRC5D is significantly elevated in multiple myeloma (MM) cells, and therefore, it is considered a potential therapeutic target for MM. Immunohistochemistry studies have shown that GPRC5D is universally expressed on malignant myeloma cells, with a distribution similar but independent to BCMA, and its expression in normal tissues is limited to hair follicles. Based on this, researchers have designed a CAR-T cell therapy containing seven human scFvs targeting GPRC5D. This therapy can eliminate MM cells in a xenograft MM mouse model and achieve long-term survival in these mice. After the infusion of GPRC5D-CAR-T cells, the mice maintained a 100% survival rate at 100 days, without any side effects such as hair loss or skin damage. This study provides important preclinical evidence for considering GPRC5D as a crucial clinical target for immunotherapy in MM.
3、Bi-specific CAR-T Cells
Although MM patients initially respond well to CAR-T cell therapy, clinical studies have found that a high proportion of patients relapse due to antigen escape, which may be related to insufficient expansion and persistence of CAR-T cells in vivo, loss or downregulation of tumor-associated antigens, and the presence of immunosuppressive factors in the tumor microenvironment. Due to the limited available treatment options, most relapsed patients have a poor prognosis. The development of multi-targeted CAR-T cells, especially bi-specific CAR-T cells, is considered the most promising solution to address antigen escape, particularly the clinical development of BCMA/CD19 bi-specific CAR-T cells.
Theoretically, bi-specific CAR-T cells can be achieved through the following four approaches:
1) As shown in Figure 3A, Cocktail/Sequential infusion, which involves the combined treatment of two mono-specific CAR-T cell populations (also known as cocktail therapy). The drawback of this approach is that the two different CAR-T cell populations may expand at different rates in vivo, ultimately leading to an imbalance in their proportions and compromising the ability to effectively eliminate tumor cells.
2) As shown in Figure 3B, Co-transduction, where two viral vectors encode two mono-specific CAR molecules, respectively, and are co-transduced into T cells to generate bi-specific CAR-T cells. However, due to differences in transduction efficiency, this approach results in an unbalanced ratio of the two CAR molecules on the CAR-T cells, and some T cells may only express one CAR molecule.
3) As shown in Figure 3C, Bicistronic CAR-T cells, where a single viral vector encodes two mono-specific CAR molecules, resulting in CAR-T cells expressing two CAR molecules. This approach generates bi-specific CAR-T cells with equal ratios of the two CAR molecules. However, the limitation is the limited packaging capacity of the viral vector, leading to lower expression levels and potential incomplete expression of the second CAR molecule.
4) As shown in Figures 3D-E, Bivalent CAR-T cells, where a single viral vector encodes a CAR molecule with two antigen-binding domains. The bivalent CAR structure can be designed in a linear tandem configuration or a loop configuration. These two bivalent CAR formats are currently the hottest research areas and the most widely applied bi-specific CAR-T cell designs.
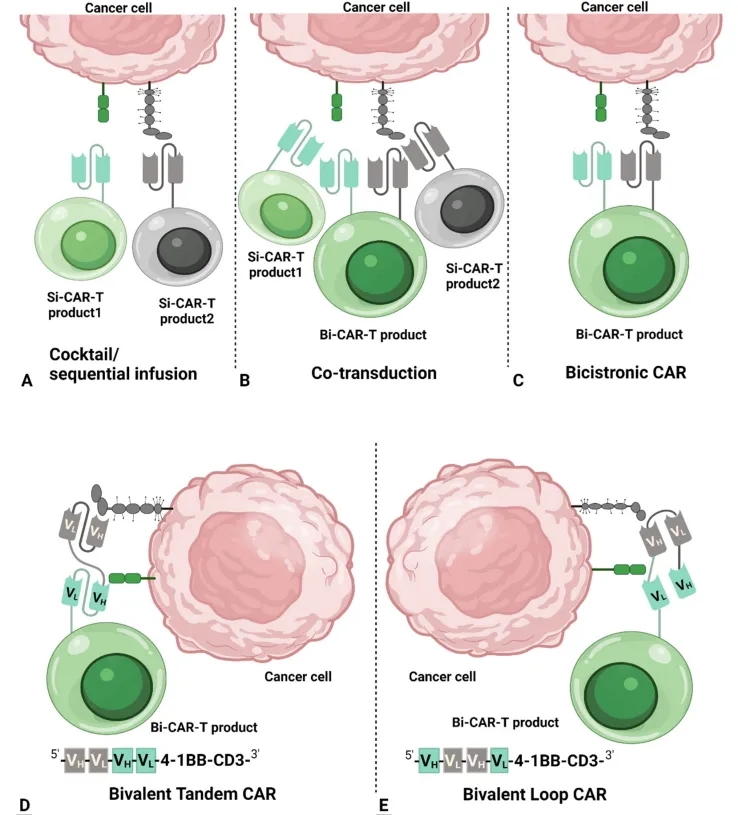
Figure 3: Schematic representation of bi-specific CAR designs
Compared to traditional therapeutic approaches such as proteasome inhibitors, monoclonal antibodies, and immunomodulators, CAR-T cell therapy offers greater specificity and potent cytotoxicity, leading to improved remission rates and survival for patients. However, relapse due to tumor immune evasion remains a significant challenge. Currently, clinical studies on multi-targeted, particularly bi-specific, CAR-T cells have shown promising results in addressing this issue. With further research and clinical trials, the safety and efficacy of CAR-T cell therapy are expected to improve, bringing more hope to patients with multiple myeloma.
References
[1] VAN DE DONK N W C J,PAWLYN C,KL Y.Multiple mye-lomalJ]. Lancet,2021, 397(10272):410-427.
[2] Kagoya, Y.; Tanaka, S.; Guo, T.; Anczurowski, M.; Wang, C.H.; Saso, K.; Butler, M.O.; Minden, M.D.; Hirano, N. A novel chimeric antigen receptor containing a JAK-STAT signaling domain mediates superior antitumor effects. Nat. Med. 2018, 24, 352–359.
[3] NOOPUR RAJE M D J B. Anti-BCMA CAR T-Cell Therapybb2121 in Relapsed or Refractory Multiple Myeloma[J]. TheNew England Journal of Medicine, 2019(380) :1726-1737.
[4] LIN Y,MARTIN T,BERDEJA J G,et al. Ciltacabtagene autoleucel, a BCMA-directed CAR-T cell therapy, in patients withrelapsed/refractory multiple myeloma: 2-year post LPI resultsfrom the phase 1b/2 CARTITUDE-1 study[J]. HemaSphere,2022,6(Suppl) :851-852.
[5] Hsi ED, Steinle R, Balasa B, et al. CS1, a potential new therapeutic antibody target forthe treatment of multiple myeloma. Clin Cancer Res. 2008;14(9):2775-2784.
[6] Smith E L, Harrington K, Staehr M, et al. GPRC5D is a target for the immunotherapy of multiple myeloma with rationally designed CAR T cells[J]. Science translational medicine, 2019, 11(485): eaau7746.
[7] SHAH N N,MAATMAN T,HARI P,et al. Multi TargetedCAR-T Cell Therapies for B-Cell Malignancies[J]. Frontiers inOncology,2019,9:146.
[8] TAHMASEBI S,ELAHI R,KHOSH E,et al. Programmableand multi-targeted CARs: a new breakthrough in cancer CAR-Tcell therapy[J]. Clinical and Translational Oncology, 2021, 23(6):1003-1019.
[9] Xie, B.; Li, Z.; Zhou, J., Wang, W., Current status and perspectives of dual-targeting chimeric antigen receptor T-cell therapy for the treatment of hematological malignancies. Cancers 2022, 14.
Content Source:深圳细胞谷
