The ddBCMA CAR-T (Anitocabtagene Autoleucel, Anito-cel) with a Remarkable 100% ORR: The Dawn Finally Breaks for this CAR-T Therapy with a Turbulent Journey
The ddBCMA CAR-T (Anitocabtagene Autoleucel, Anito-cel) with a Remarkable 100% ORR: The Dawn Finally Breaks for this CAR-T Therapy with a Turbulent Journey
Preface
On December 8, 2023, Arcellx released new clinical data from the phase 1 expansion study of CAR-T-ddBCMA [now called anitocabtagene autoleucel (anito-cel)].
The latest data demonstrated the durable long-term remission of anito-cel, and as of the data cutoff date of October 15, 2023, the median duration of remission, progression-free survival (PFS), and overall survival (OS) were not reached.
In the phase 1 expansion study of anito-cel, the median follow-up time was 26.5 months, with 38 evaluable patients. According to the International Myeloma Working Group (IMWG) criteria, all evaluable patients achieved an overall response rate (ORR) of 100%. Although it was only a phase 1 expansion study, a 100% ORR is still an excellent achievement.
However, despite such remarkable results, the development path of anito-cel has not been a smooth one…
1. A stumble
When anito-cel was still called CART-ddBCMA, it was indeed favored by everyone.
On December 9, 2022, Gilead’s Kite company reached an agreement with Arcellx to jointly develop the BCMA CAR-T product anito-cel, with an upfront payment of $225 million and up to $3.9 billion in milestone payments, totaling $4.125 billion.
For a product still in phase 2 clinical trials, $4.125 billion was not a low price. Gilead, the buyer of anito-cel, was clearly not doing charity work; it was willing to pay a high price for this CAR-T product because anito-cel’s early data was exceptionally promising.
As a therapeutic target for multiple myeloma, currently, only two BCMA CAR-T products have been approved for marketing: Legend Biotech/Johnson & Johnson’s Ciltacabtagene Autoleucel and BMS/Bluebird bio’s Abecma. Between these two products, Ciltacabtagene Autoleucel has superior data in various aspects.
Anito-cel’s early data was so impressive that, compared to the current BCMA CAR-T leader Ciltacabtagene Autoleucel, its data was not inferior and even showed a trend of surpassing the leader.
Let’s compare the data in detail:
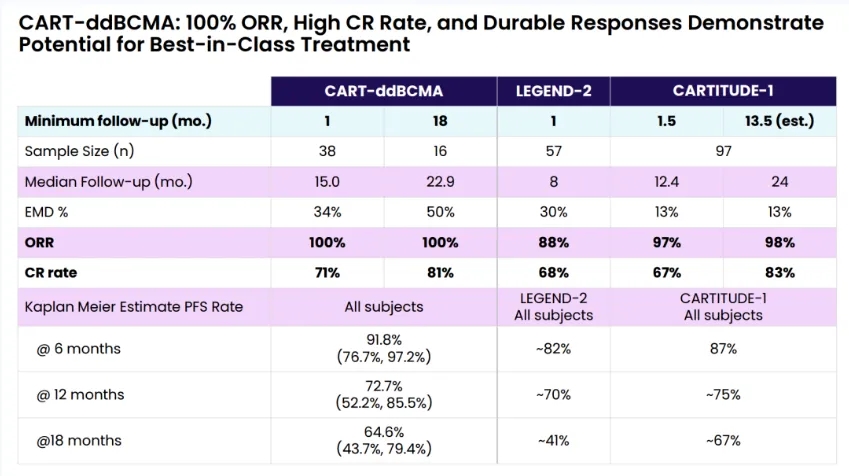
When the early clinical trials LEGEND-2 and CARTITUDE-1 of Ciltacabtagene Autoleucel were announced, they generated tremendous excitement. However, when compared to anito-cel, we can see that except for the CR rate at 24 months of follow-up in the CARTITUDE-1 trial, which was slightly higher, the remaining data favored anito-cel.
It’s also worth noting the patient populations included in the trials.
In the CARTITUDE-1 trial, the proportions of triple-refractory and penta-refractory patients were 88% and 42%, respectively, while anito-cel increased these proportions to 100% and 68%.
This data indicates that anito-cel selected more difficult-to-treat patients, yet still achieved better study results on this basis.
Such exceptional data is not undeserving of the high price Gilead paid to acquire this product from Arcellx. After all, everyone could foresee that the commercialization of anito-cel would mark the coronation of a new king in the CAR-T field, with no need to worry about its commercial performance.
Unfortunately, life is unpredictable, and anito-cel stumbled before ascending to the throne…
2. Hitting a landmine
With the outstanding phase 1 study data, Arcellx’s ambitions were also growing. According to Arcellx’s plan, they intended to submit a Biologics License Application (BLA) to the FDA for anito-cel directly after completing the phase 2 clinical trial iMMagine-1, aiming for accelerated approval.
Unfortunately, Arcellx did not get the final results of the iMMagine-1 study. Due to the sudden death of a patient, the iMMagine-1 study was halted by the FDA.
According to Arcellx’s disclosure, the cause of the patient’s death might have been due to a deficiency in the bridging therapy. However, apart from the choice of treatment regimen, the more fundamental reason could be the excessively challenging clinical trial design.
Gilead and Arcellx might have intended to enroll more difficult-to-treat patients to obtain higher-quality clinical data for a faster approval, but this backfired.
Let’s again use Ciltacabtagene Autoleucel as a reference to understand the purpose of the iMMagine-1 study.
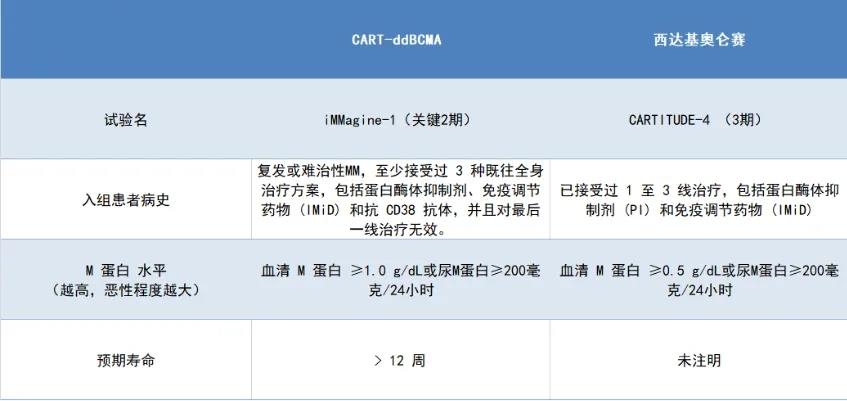
Some inclusion criteria that are not on the same level have not been compared here; only a few comparable criteria have been selected. However, the result is still evident: Arcellx aimed to enroll patients with more severe conditions.
Based on the phase 1 study results of anito-cel, Arcellx’s approach was justifiable. They had full confidence in anito-cel and hoped for its rapid market entry. Additionally, according to Arcellx’s introduction, their technology also had certain advantages.
Arcellx designed a new class of D-Domain-driven autologous and allogeneic CAR-T cells to overcome some limitations of traditional cell therapy approaches, including classical single-dose CAR-T (termed ddCARs).
As described by Arcellx, the D-Domain is a small, stable, fully synthetic conjugate with a hydrophobic core. When used for CARs, its unique structure may enable higher transduction efficiency, higher cell surface expression, and lower tonic signaling, designed to improve target specificity while enhancing binding affinity.
The ddCAR consists of an intracellular T-cell signaling domain similar to a traditional CAR, combined with a D-Domain as the extracellular antigen-binding region. In other words, by adopting the novel synthetic binding scaffold D-Domain instead of the scFv as the antigen-binding domain in CAR-T cells.
With superior technology and product, Arcellx took a reckless step, but failed to achieve the desired result. After all, there are too many uncertainties in clinical trials, such as the failure reason provided by Arcellx this time—bridging therapy.
Due to the highly personalized and customized nature of CAR-T cell therapy, patients need 3-4 weeks to prepare the treatment after confirming their acceptance. During this period, bridging therapy is needed to prevent disease progression.
Since bridging therapy is a temporary treatment without standardized methods, it entirely depends on the patient’s treatment history, previous treatment toxicity and resistance, presence of specific comorbidities, tumor burden, and the expected timing of CAR-T cell infusion, among other factors, exhibiting significant individual tendencies.
However, in clinical trials, uncertainties are like landmines, which can explode unexpectedly. Unfortunately, Arcellx stepped on one of these landmines…
3. Rallying
In August 2023, the FDA finally brought good news by lifting the clinical hold on anito-cel. The FDA not only signed off on the updated trial protocol but also agreed to expand the scope of bridging therapies.
Fortunately, although severely impacted, Arcellx did not intend to give up on anito-cel, this potential “cash cow.”
Based on the latest data, Arcellx’s persistence has paid off, as the phase 1 expansion study results have brought anito-cel back into the spotlight.
In addition to the 100% ORR, as of the data cutoff, among the 38 evaluable patients in this phase 1 expansion trial of anito-cel, 29 achieved complete remission (CR) or stringent complete remission (sCR) (>CR rate, 76%); 35 achieved very good partial remission (VGPR) or better (>VGPR rate, 92%).
The median PFS has not been reached in the anito-cel phase 1 expansion study, but according to the Kaplan-Meier estimate, the PFS rates at 6, 12, 18, and 24 months were 92%, 76%, 64%, and 56%, respectively.
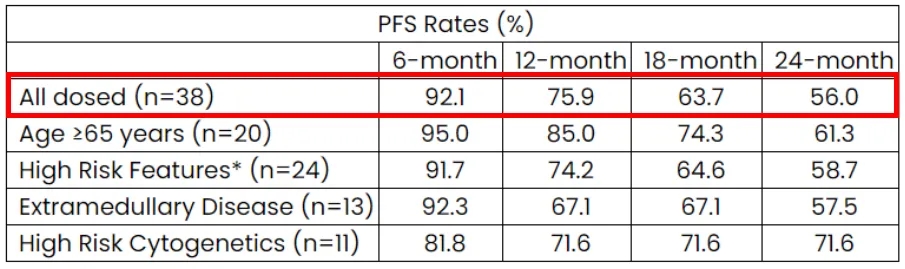
Additionally, regarding the highly anticipated safety profile of anito-cel, it provided a satisfactory answer.
As of the data cutoff, at the RP2D dose (1.15 billion (+/-10) CAR+ T cells), anito-cel demonstrated good tolerability, with manageable adverse events in the trial, including cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS). No grade 3 or higher CRS events occurred, and only one case of grade 3 ICANS (3%) was observed.
Furthermore, no on-target tissue toxicity or delayed neurotoxicity events or parkinsonism were observed in the trial.
The phase 1 expansion trial of anito-cel has allowed it to regain its luster. Currently, Arcellx is planning to register the iMMagine-1 study for anito-cel and recruit patients.
We do not know whether Arcellx, who previously witnessed anito-cel stumble due to the high-difficulty trial design, will continue their previous approach and conduct another high-difficulty clinical trial to accelerate anito-cel’s approval.
However, Arcellx has previously taken a significant risk, and now they have faced failure. Regardless of Arcellx’s next steps, they have already become the creators of a new Legend Biotech generation.
Reference Material
1.Arcellx官网
2.https://ir.arcellx.com/news/news-details/2023/Arcellx-Announces-Continued-Robust-Long-Term-Responses-from-Its-CART-ddBCMA-anito-cel-Phase-1-Expansion-Trial-in-Patients-with-Relapsed-or-Refractory-Multiple-Myeloma-at-ASH/default.aspx
3.https://mp.weixin.qq.com/s/G_0bN4_3sYA8Hhm4mN5sFA
4.https://clinicaltrials.gov/
5.https://www.fiercebiotech.com/biotech/fda-lifts-clinical-hold-gileads-car-t-arcellx-sheds-light-reason-patient-death
6.其他公开资料
Content Source:药时代服务号
