Drug Resistance Mechanism and Clinical Significance of Bispecific T Cells and Multiple Myeloma Therapy
Drug Resistance Mechanism and Clinical Significance of Bispecific T Cells and Multiple Myeloma Therapy
Bispecific T Cell Engagers (TCEs) for Multiple Myeloma (MM)
Bispecific T cell engagers (TCEs) are revolutionizing the treatment of multiple myeloma (MM) patients by redirecting T cells against cancer cells. Teclistamab and elranatamab, targeting CD3 and BCMA, have been approved overseas for the treatment of three classes of relapsed/refractory multiple myeloma (RRMM), based on the Majestec-1 and Magnetismm-3 studies, respectively. Additionally, bispecific TCEs targeting other tumor antigens (e.g., FCRH5 and GPRC5D) have also demonstrated promising efficacy, with talquetamab, a bispecific TCE targeting GPRC5D, approved for the treatment of RRMM (based on the MONUMENTAL-1 study).
Bispecific TCEs are also being investigated in early-stage treatment, including first-line therapy. However, approximately one-third of RRMM patients exhibit primary resistance in clinical trials, and most responders eventually develop acquired resistance. Therefore, understanding the mechanisms of resistance to bispecific TCEs is crucial for improving immunotherapy for MM.
A recent review published in Blood Advances elucidates the tumor-intrinsic and tumor-extrinsic mechanisms contributing to bispecific TCE resistance and their clinical implications.

Clinical determinants of TCE resistance
Clinical trials have identified multiple baseline clinical factors that predict poor response to BCMA BsAbs, including the presence of extramedullary disease (EMD), International Staging System (ISS) stage III, and refractory disease status. In the Magnetismm-3 study, patients with EMD had an overall response rate (ORR) of 38.5% to elranatamab, compared to 71.4% in those without EMD. Patients with ISS stage III (compared to ISS stages I-II) and penta-refractory disease (compared to non-penta-refractory) also had lower response rates to elranatamab. In the Majestec-1 study, patients with EMD or ISS stage III also had significantly lower ORRs to teclistamab. The lower response rate in EMD patients to teclistamab may be related to higher levels of soluble BCMA in this population. High tumor burden was also associated with lower response rates to elranatamab (bone marrow [BM] plasma cells >50%) and teclistamab (BM plasma cells >60%). However, high-risk cytogenetics did not significantly impact response rates to either drug.
Talquetamab is the only approved GPRC5D BsAb to date. In the Monumental-1 study, extramedullary disease was the only baseline clinical feature that significantly affected response rates. Patients with EMD had median ORRs of 48.5% and 43.2% in the once-weekly and once-every-two-weeks cohorts, respectively, while those without EMD had median ORRs of 81.8% and 88%, respectively. ISS, cytogenetics, and refractory status did not significantly impact response rates to talquetamab.
Tumor-intrinsic mechanisms of TCE resistance
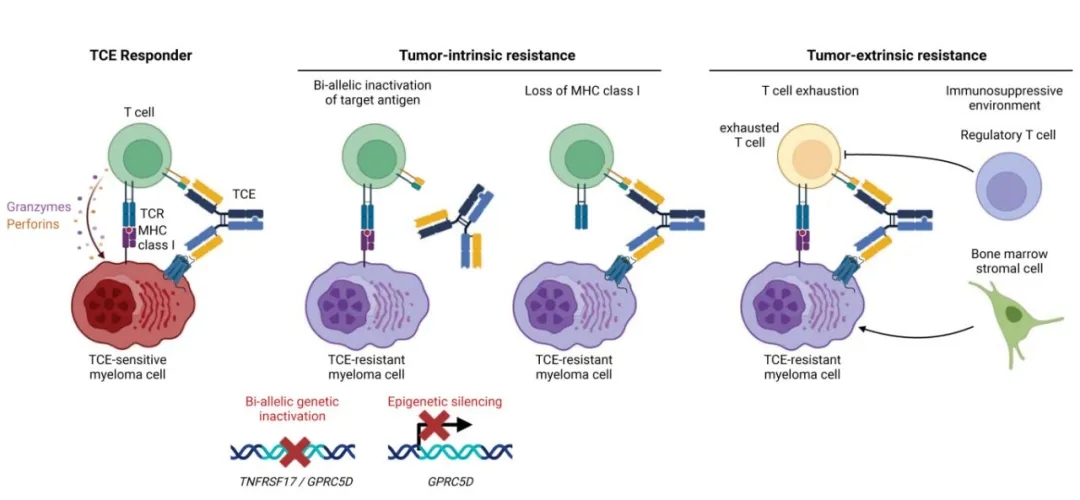
Tumor-intrinsic mechanisms of TCE resistance include TNFRSF17 gene inactivation, GPRC5D genetic or epigenetic inactivation, and loss of major histocompatibility complex class I (MHC I) molecules.
Whole-genome sequencing (WGS) of myeloma cells before and after BCMA TCE treatment has revealed that inactivation of the TNFRSF17 gene (encoding the BCMA protein) is a common tumor-intrinsic resistance mechanism.
The mutational landscape of GPRC5D involves truncating mutations distributed along the protein sequence, in contrast to the hotspot mutations observed in TNFRSF17, which can alter TCE recognition while retaining BCMA-mediated pro-survival signals (Figure 1). Resistance to GPRC5D TCEs typically involves complete inactivation of the target, suggesting that myeloma cells are more tolerant of GPRC5D deficiency than BCMA deficiency.
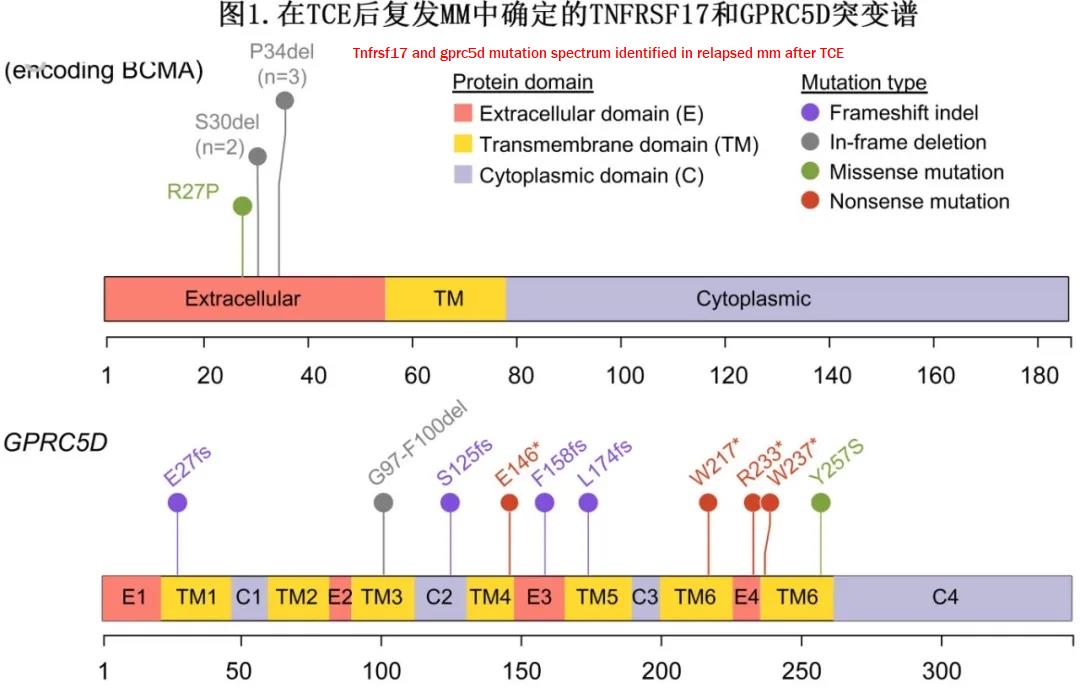
Multiple lines of evidence suggest that loss of MHC class I molecules may be a potential tumor-intrinsic mechanism of TCE resistance in the context of target antigen loss. First, dysregulation of MHC class I (HLA-E, HLA-C) and class II (CD74) genes has been observed after TCE treatment. Second, loss of surface expression of MHC class I molecules has been detected by flow cytometry in some patients at relapse. However, the frequency and causal mechanisms of MHC class I molecule expression loss remain to be determined.
Tumor-extrinsic resistance mechanisms
The response to bispecific TCE therapy is influenced by various tumor-extrinsic factors, including the pre-existing T cell landscape, the evolution of the T cell landscape, and the immunosuppressive tumor microenvironment generated by myeloma cells, which can be related to prior treatments.
Figure 2 summarizes the tumor-intrinsic and tumor-extrinsic mechanisms of TCE resistance.
Clinical implications
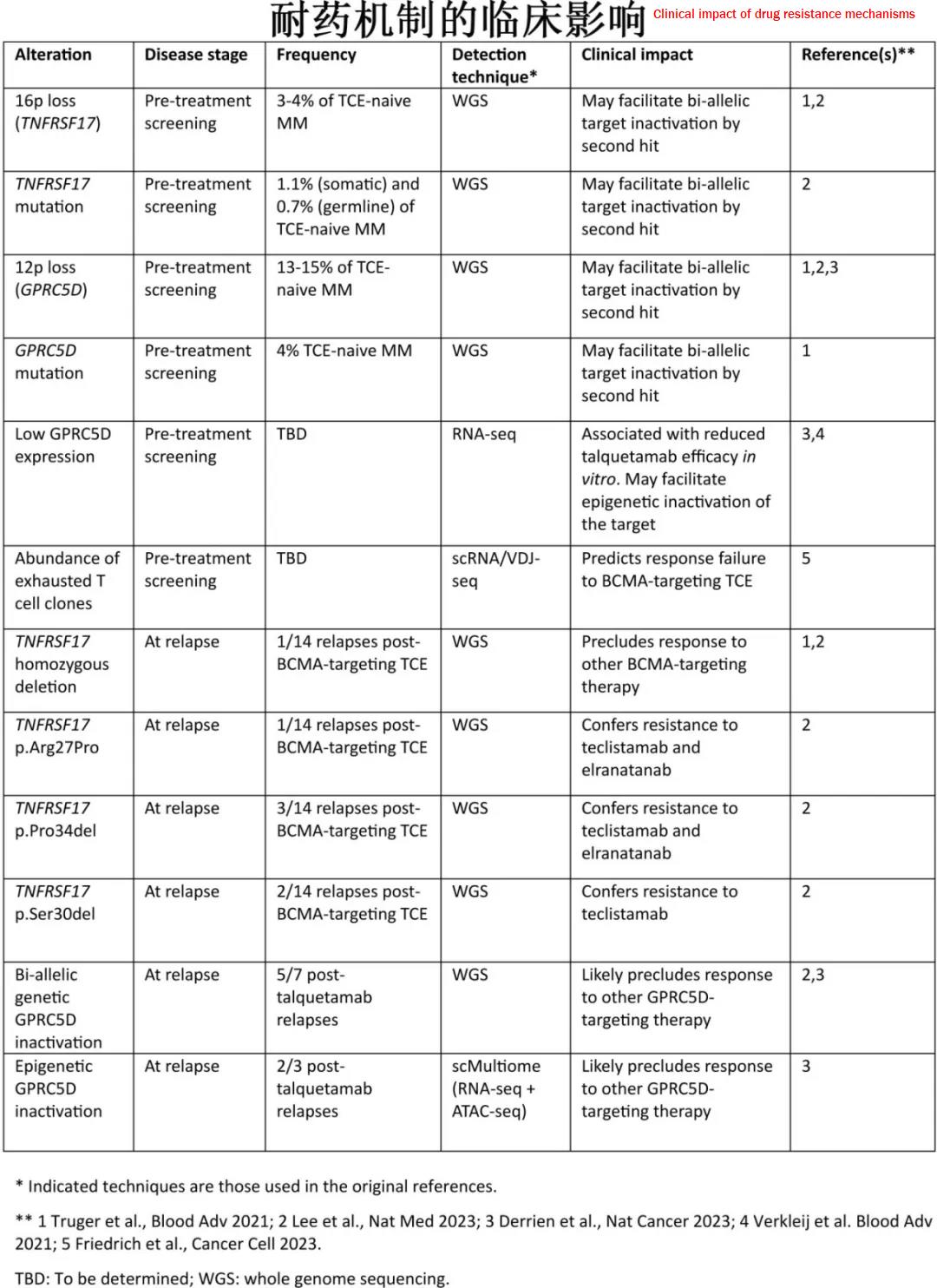
Elucidating the molecular mechanisms of TCE resistance can provide valuable insights for guiding future immunotherapy for multiple myeloma. Characterizing the molecular features of the tumor cells and the immune cell repertoire before treatment can aid in selecting the most appropriate immunotherapy for each patient. Understanding the molecular basis of resistance at relapse can help guide the selection of subsequent lines of treatment and the sequencing of immunotherapies.
Summary
This article reviews recent studies on the clinical and molecular factors contributing to bispecific TCE resistance, which can arise from tumor-intrinsic or tumor-extrinsic mechanisms. Tumor-intrinsic resistance includes various alterations leading to target antigen loss, such as chromosomal deletions, point mutations, or epigenetic silencing. The loss of MHC class I molecules, which can impair the MHC class I:TCR co-stimulatory signal, has also been reported. Tumor-extrinsic resistance mechanisms include an enriched repertoire of exhausted T cell clones and multiple factors contributing to an immunosuppressive microenvironment. Importantly, some resistance mechanisms impair the response to one TCE while preserving the efficacy of others. These findings have clinical implications, as monitoring the status of the target antigen in tumor cells and their immune microenvironment will be crucial for selecting the most appropriate TCE for each patient and designing combination and sequencing strategies for immunotherapy in multiple myeloma.
References
Letouzé E, et al. Mechanisms of resistance to bispecific T cell engagers in multiple myeloma and their clinical implications.Blood Adv . 2024 Mar 21:bloodadvances.2023012354. doi: 10.1182/bloodadvances.2023012354.
Content Source:无癌界
