Overview of BCMA in Multiple Myeloma CAR-T Cell Therapy
Overview of BCMA in Multiple Myeloma CAR-T Cell Therapy
Introduction to BCMA
BCMA (B-cell maturation antigen) is considered a major target for the treatment of multiple myeloma. Currently, the three most commonly used therapeutic approaches targeting BCMA are bispecific antibody constructs, such as antibody-drug conjugates (ADCs), BiTE® (bispecific T-cell engagers), immunoconjugate therapies, and CAR-modified T-cell therapies.
CAR T-cell-mediated BCMA therapy has demonstrated clinical efficacy in multiple myeloma. This review article provides an overall overview of all available CAR T-cell-mediated BCMA therapies in the treatment of multiple myeloma.
BCMA (B-cell Maturation Antigen)
BCMA, also known as CD269 or TNFRSF17, is a protein encoded by the TNFRSF17 gene, which is 2.92 kilobases long and is transcribed on the short arm (16p13.13) of chromosome 16. It consists of three exons and two introns. BCMA is a 184-amino-acid peptide, a type III transmembrane glycoprotein of 20.2 kDa, with a conserved six-cysteine motif at the extracellular N-terminus. It belongs to the human tumor necrosis factor receptor (TNFR) superfamily.
BCMA has four natural splice variants with different receptor-binding affinities, intracellular signaling domains, and membrane-anchoring capabilities. It binds two ligands, APRIL and BAFF, which are primarily produced in the bone marrow by osteoclasts, stromal cells, and macrophages in a paracrine manner. It is primarily expressed on mature B lymphocytes, with lower levels in non-hematopoietic tissues and hematopoietic stem cells.
BCMA interacts with the other two functional subunits of the TNFR superfamily, BAFF, which is essential for the survival of long-lived bone marrow plasma cells. APRIL is a proliferative stimulatory ligand with a higher affinity for BCMA than BAFF (Figure 1). It binds to TACI, while BAFF has a higher affinity for BAFF-R (Figure 1). BCMA is predominantly expressed on plasma cells and plasmablastic cells. Furthermore, low levels are detected on memory B cells and plasmablastic dendritic cells committed to PC development. Apart from certain areas of the human body, including the trachea, testes, and certain parts of the gastrointestinal tract, BCMA is undetectable in immature B cells, normal non-hematopoietic tissues, or hematopoietic stem cells.
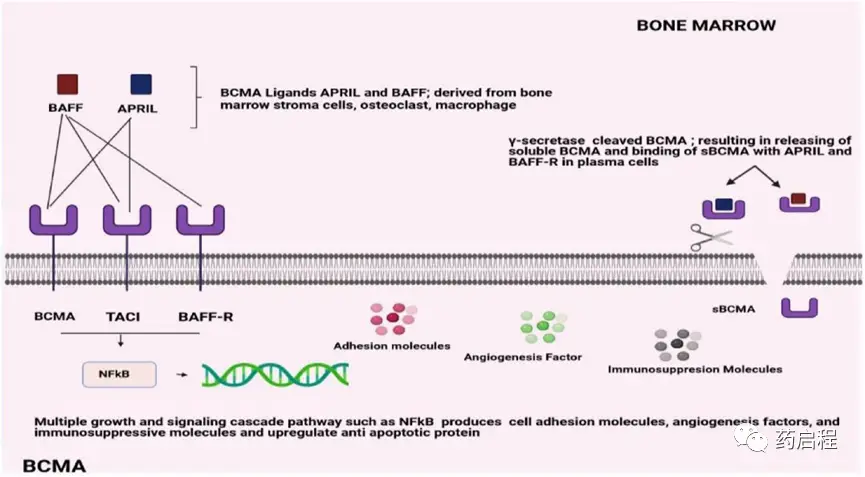
Figure 1. Structural overview of BCMA binding affinities
Functions and Expression of BCMA
The BCMA surface protein, commonly referred to as CD269 or TNFRSF17, is initially expressed on late-stage memory B cells and subsequently frequently observed on plasma cells. BAFF and APRIL are the two B-cell activation and proliferation-inducing ligands that bind to BCMA. Consequently, multiple myeloma cells activate numerous growth and survival signal cascades, most notably NF-kB, as well as the PI3K-PKB/Akt and RAS/MAPK signaling pathways.
In multiple myeloma patients, serum levels of APRIL and BAFF are approximately five times higher than in healthy donors. The ligand concentration increase has been determined to correlate with the stage of myeloma progression. Multiple myeloma cells have been shown to promote the release of more APRIL by osteoclasts, thereby reducing the efficacy of the immune system against the BM microenvironment. It has been reported that the addition of an APRIL-blocking monoclonal antibody (mAb) to B-cell maturation antigen-targeted immunotherapy may help alleviate the immunosuppressive BM environment induced by MM cells, thus enhancing antibody-dependent cell-mediated cytotoxicity (ADCC) against multiple myeloma cells.
BCMA is essential for the maturation and survival of long-lived plasma cells but may be less important for overall humoral B-cell responses. BCMA is frequently expressed on multiple myeloma cells at various epitope densities and has been shown to promote MM development and reduce immune system activation in the BM microenvironment. Membrane-bound BCMA can be cleaved by γ-secretase, leading to a decrease in overall cell surface BCMA expression and the formation of a soluble form (sBCMA), which interferes with the binding of therapeutic compounds to BCMA.
Notably, there is no evidence of substantial BCMA expression in non-hematological areas. In mouse models, BCMA overexpression significantly enhanced the in vivo proliferation of xenografted multiple myeloma cells. Furthermore, BCMA expression is upregulated in the pathophysiology and progression of multiple myeloma, from monoclonal gammopathy of undetermined significance to active MM, although its significance is uncertain. Elevated BCMA levels are associated with poorer outcomes, suggesting that BCMA may be an important indicator of multiple myeloma disease activity and prognosis.
In vitro, BCMA overexpression in multiple myeloma cells activates the NF-κβ and MAPK pathways, which do not require APRIL/BAFF activation. Additionally, there are numerous interactions between the APRIL or BCMA signaling pathways and other pathways. For example, APRIL promotes the survival and rapid proliferation of myeloma cells by interacting with HSPG (also known as CD138/syndecan-1 and sulfated glycosaminoglycan). BCMA expression is downregulated when FGF-R3 and JAK2 are simultaneously inhibited. Furthermore, an in vitro study demonstrated that BCMA co-immunoprecipitated with a master transcription factor (interferon regulatory factor-4; IRF-4) involved in MM cell survival, emphasizing the importance of BCMA in MM tumorigenesis.
BCMA: A Potential Target for Cancer Therapy
Therefore, additional biomarkers are needed for the diagnosis and monitoring of multiple myeloma. Compared to normal bone marrow mononuclear cells from healthy individuals, the B-cell maturation antigen exhibits extremely high expression on malignant PCs isolated from MM patients. Several studies have examined its value as a diagnostic, prognostic, and/or predictive biomarker for clinical outcomes. Compared to CD138, BCMA is typically observed in delayed or delayed multiple myeloma frozen samples.
The volume of membrane-bound BCMA can be quantified using flow cytometry and immunohistochemistry; however, the measurement of BCMA volume may vary across studies due to methodological differences. Interestingly, in newly diagnosed multiple myeloma and refractory/relapsed multiple myeloma patients, malignant PCs express comparable levels of BCMA mRNA, suggesting that BCMA may be a viable therapeutic target throughout the MM disease stage. BCMA has a soluble form, called soluble BCMA (sBCMA), which is directly shed from membrane BCMA through γ-secretase activity. The extracellular domain and part of the transmembrane region are retained in sBCMA.
CAR T-cell Therapy Targeting BCMA
CAR T cells are engineered T cells that express a CAR (chimeric antigen receptor) targeting a specific tumor-associated antigen, which upon engagement activates the T cells in an MHC-independent manner. These CAR constructs consist of a TAA-targeting scFv (typically murine or humanized) coupled to a CD3ζ intracellular signaling domain via an extracellular spacer and transmembrane region, as well as co-stimulatory domains (such as CD28, OX40, 4-1BB, etc.).
Over the past five years, BCMA-targeting CAR T-cell therapies have shown a revolutionary change in cancer treatment. In multiple myeloma, several anti-BCMA CAR T-cell therapies have demonstrated impressive clinical activity, achieving 90-100% response rates, and have been developed and are undergoing preclinical and/or clinical investigation. BCMA-targeting CAR T-cell therapies can be categorized into autologous BCMA, allogeneic BCMA, bispecific, BCMA-CS1 CCAR T cells, APRIL CAR T cells, BCMA CAR T cells with tEGFR, P BCMA 101, CT053, BCMA-targeting CAR-NK cells, and others. The engineered CAR-T cells release chimeric antigen receptors that can readily bind to the BCMA receptor and enhance anti-tumor activity (Figure 2).
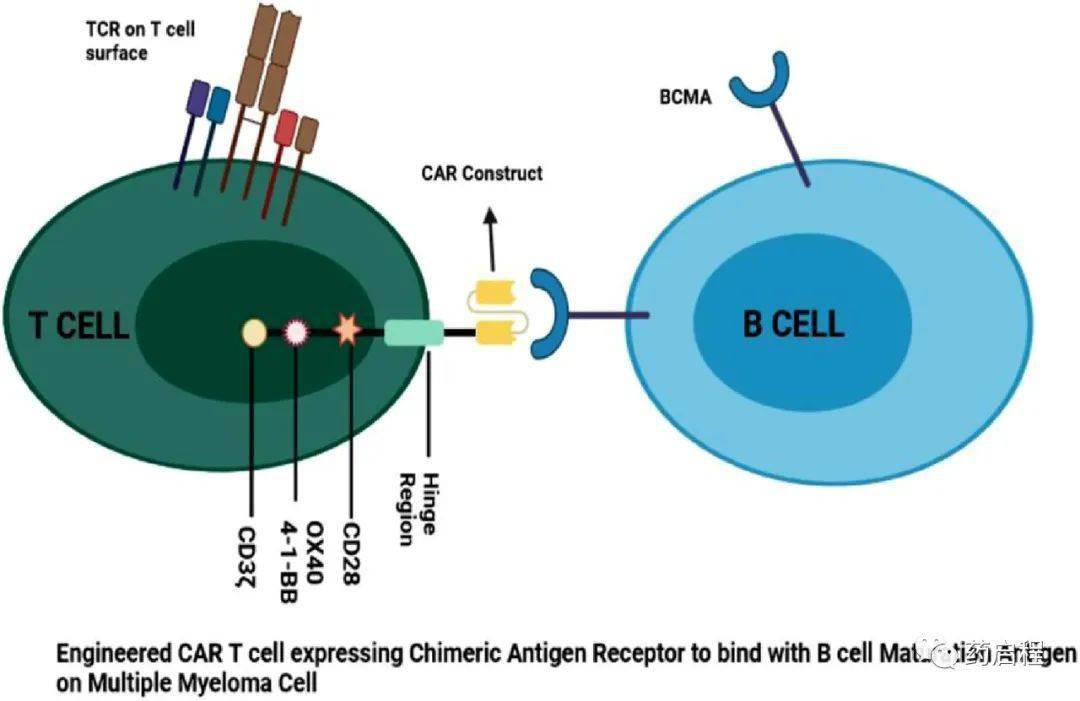
Figure 2. Schematic illustration
