Abecma (Ideabtagene Vicleucel) FDA Approval Date and Brief Introduction of Drugs
Abecma (Ideabtagene Vicleucel) FDA Approval Date and Brief Introduction of Drugs
Overview of FDA Approval of Abecma
On March 26, 2021, Abecma® (Idecabtagene Vicleucel, ide-cel) received approval from the U.S. Food and Drug Administration (FDA) for the treatment of adult patients with relapsed or refractory multiple myeloma after four or more prior lines of therapy, becoming the first FDA-approved CAR-T cell immunotherapy targeting the B-cell maturation antigen (BCMA).
On April 4, 2024, Abecma® (Idecabtagene Vicleucel, ide-cel) received FDA approval for the treatment of adult patients with relapsed or refractory multiple myeloma after two or more prior therapies, including an immunomodulatory drug (IMiD), a proteasome inhibitor (PI), and an anti-CD38 monoclonal antibody based on the results of the KarMMa-3 trial. This approval expands the indication for Abecma, allowing its use in earlier lines for patients who have relapsed or become refractory after exposure to these three major classes of therapy (triple-class exposure), as well as after two prior therapies.
Abecma is priced at $419,500 per infusion bag.

Introduction to Abecma
Abecma (Idecabtagene Vicleucel; ide-cel) is a CAR-T cell therapy that can recognize and bind to BCMA on the surface of multiple myeloma cells, leading to CAR-T cell proliferation, cytokine secretion, and subsequent cytolytic killing of BCMA-expressing cells. Although progress has been made, multiple myeloma remains an incurable disease characterized by remissions and relapses. In early treatment, regimens typically involve combinations of IMiDs, PIs, and anti-CD38 monoclonal antibodies to help control the disease. Unfortunately, as many patients continue to relapse and/or become refractory to these classes of therapy, more patients are receiving triple-class exposure earlier in their treatment journey.
Mechanism of Action of Abecma
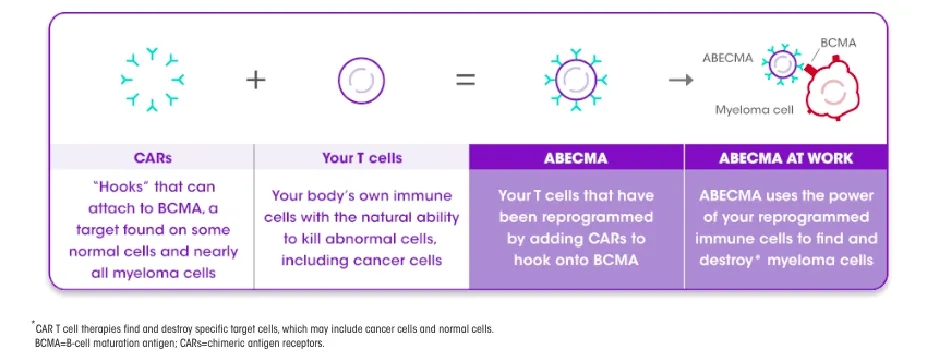
The principle of Abecma is to graft the BCMA receptor onto the patient’s T cells. The preparation process involves separating T cells from the patient’s blood, modifying the T cells using a lentiviral vector encoding the BCMA antigen receptor, and enabling the T cells to express the BCMA receptor on their surface.
During treatment, multiple myeloma patients first receive preconditioning with two chemotherapy drugs (cyclophosphamide and fludarabine) to kill existing T cells in the patient’s body, followed by the infusion of Abecma. Once infused back into the patient’s body, Abecma begins to seek out and kill cells expressing BCMA.
Clinical Data of Abecma
Abecma is indicated for the treatment of adult patients with relapsed or refractory multiple myeloma after at least four prior lines of therapy (of different types) have failed or the disease has relapsed.
For example, treatments may include immunomodulatory drugs, proteasome inhibitors, and anti-CD38 monoclonal antibodies. If these treatments are unsuccessful, patients may try Abecma.
The FDA approved Abecma based on data from the phase 2 KarMMa trial.
The evaluable population consisted of 100 patients who received Abecma treatment.
Of these patients, 88% had received four or more prior therapies, and 85% were triple-class refractory.

The study data showed an overall response rate (ORR) of 72%, with 29% of patients achieving a stringent complete response (sCR), and responses were rapid and durable.
The median time to response for complete responders was 30 days, and the median duration of response was 11 months, with a response duration of 21 months.
Of the 28 patients who achieved sCR, approximately 65% had a response lasting at least 12 months.
Safety Evaluation of Abecma
In the trial, the efficacy and safety profile was favorable, with mainly low-grade cytokine release syndrome (CRS) and neurotoxicity (NT) observed.
85% of patients experienced CRS, with 9% of patients experiencing grade 3 or higher CRS (one patient reported grade 5 CRS).
The most common manifestations of CRS included fever (98%), hypotension (41%), tachycardia (35%), chills (31%), hypoxia (20%), fatigue (12%), and headache (10%).
