Recent Updates on CAR-T Clinical Trials for Multiple Myeloma
Recent Updates on CAR-T Clinical Trials for Multiple Myeloma
Multiple myeloma is a blood cancer where plasma cells proliferate in an uncontrolled manner. Patients with this incurable cancer exhibit non-specific symptoms. These patients receive stem cell transplantation and chemotherapy. Multiple myeloma patients have a high risk of relapse.
On April 5, 2024, the U.S. Food and Drug Administration (FDA) approved ciltacabtagene autoleucel (Carvykti) for the treatment of relapsed or refractory multiple myeloma after one or more lines of therapy [1]. This therapy can be used for patients who are refractory to lenalidomide and have previously received immunomodulatory agents and proteasome inhibitors.
Ciltacabtagene autoleucel is a chimeric antigen receptor (CAR) T-cell therapy targeting B-cell maturation antigen (BCMA), a transmembrane glycoprotein expressed on malignant plasma cells [2]. CAR T-cell therapy is prepared by genetically modifying a patient’s T cells.
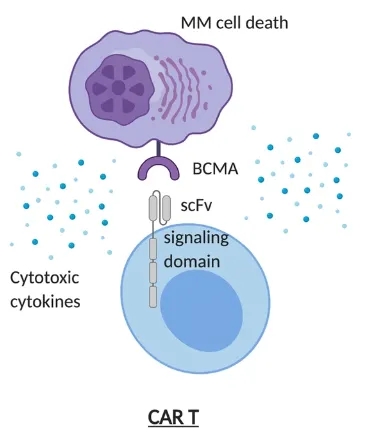
CAR T cells bind to BCMA and kill multiple myeloma cells [Source: Reference 2]
The FDA approved this therapy based on results from the phase 3 CARTITUDE-4 clinical trial. The results of this trial were presented at the 2023 American Society of Clinical Oncology (ASCO) annual meeting. In this trial, 208 multiple myeloma patients received ciltacabtagene autoleucel, and 211 patients received standard regimens. The standard regimens given were daratumumab, pomalidomide, and dexamethasone (DPd) or pomalidomide, bortezomib, and dexamethasone (PVd). Patients in the ciltacabtagene autoleucel arm received lymphodepleting chemotherapy (cyclophosphamide and fludarabine) and bridging therapy.
At a median follow-up of 15.9 months, the median progression-free survival for patients on standard regimens was 11.8 months [3]. Patients receiving ciltacabtagene autoleucel did not reach median progression-free survival. Ciltacabtagene autoleucel reduced the risk of death or disease progression by 59% compared to standard regimens. The 12-month progression-free survival rates were 75.9% and 48.6% for patients receiving ciltacabtagene autoleucel and standard regimens, respectively.
Among patients receiving ciltacabtagene autoleucel, 73.1% achieved complete response or better, compared to 21.8% in the standard regimen arm. The overall response rates were 84.6% and 67.3% for patients receiving ciltacabtagene autoleucel and standard regimens, respectively. Similarly, the minimal residual disease negativity rates were 60.6% and 15.6% for patients receiving ciltacabtagene autoleucel and standard regimens, respectively.
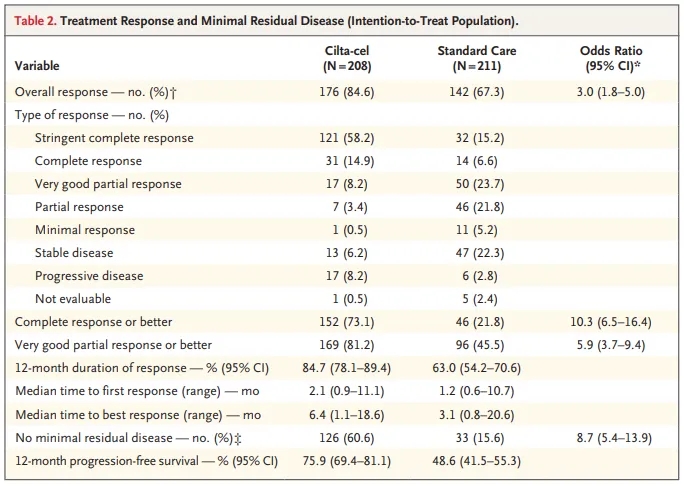
Treatment response in patients receiving standard regimens and ciltacabtagene autoleucel [Source: Reference 3]
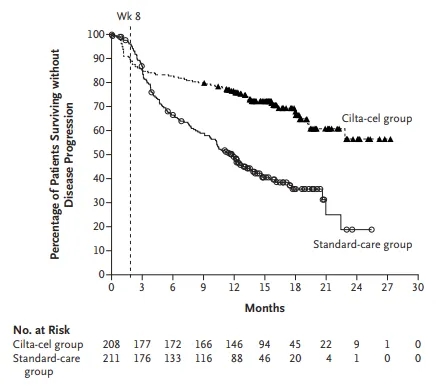
Progression-free survival in patients receiving standard regimens and ciltacabtagene autoleucel [Source: Reference 3]
Safety was evaluated in 208 patients in each of the ciltacabtagene autoleucel and standard regimen arms. Grade 3 or 4 adverse events occurred in 96.6% and 94.2% of patients receiving ciltacabtagene autoleucel and standard regimens, respectively. Similarly, 44.2% and 38.9% of patients receiving ciltacabtagene autoleucel and standard regimens, respectively, experienced serious adverse events. Common grade 3 or 4 adverse events in patients receiving ciltacabtagene autoleucel included thrombocytopenia, hypogammaglobulinemia, neutropenia, and anemia. This therapy carries a black box warning for complications such as hemophagocytic lymphohistiocytosis/macrophage activation syndrome (HLH/MAS), cytokine release syndrome, and Graft-versus-Host Disease.
In 2022, the FDA approved this therapy for relapsed or refractory multiple myeloma patients after four or more prior lines of therapy, based on results from the phase 1b/2 CARTITUDE-1 trial [4]. In this trial, 97 relapsed or refractory multiple myeloma patients received this treatment. The overall response rate was 97.9% [5]. The median duration of response for responders was 21.8 months [6].
On April 4, 2024, the FDA approved idecabtagene vicleucel for the treatment of multiple myeloma patients after at least two prior therapies. Idecabtagene vicleucel is another CAR T-cell therapy targeting BCMA. This therapy can be used in patients previously treated with an anti-CD38 antibody, a proteasome inhibitor, and an immunomodulatory agent. The FDA approved this based on the phase 3 KarMMa-3 trial. In this trial, 254 patients received idecabtagene vicleucel, and 132 received standard regimens [7]. Standard regimens included DPd, elotuzumab, pomalidomide, and dexamethasone (EPd), daratumumab, bortezomib, and dexamethasone (DVd), carfilzomib and dexamethasone (Kd), or isatuximab, lenalidomide, and dexamethasone (IRd) [8].
The median progression-free survival was 13.8 months and 4.4 months for patients receiving idecabtagene vicleucel and standard regimens, respectively. This therapy reduced the risk of death or disease progression by 51% compared to standard therapy. The overall response rate was 71.3% in patients receiving idecabtagene vicleucel. 43.7% achieved stringent complete/complete response. In contrast, the overall response rate was 42.4% in patients receiving standard regimens, with 5.3% achieving stringent complete/complete response. The median overall survival was 41.4 months and 37.9 months for patients receiving idecabtagene vicleucel and standard regimens, respectively.
In 2021, the FDA approved this therapy for multiple myeloma patients after at least four prior lines of therapy, based on the phase 2 KarMMa trial [9]. In this trial, 127 multiple myeloma patients who had received three or more prior lines of therapy received idecabtagene vicleucel [10]. The overall response rate was 72%, with 28% achieving stringent complete response. The median duration of response for all responders was 11 months.
In summary, the FDA has approved ciltacabtagene autoleucel for second-line treatment of multiple myeloma. Idecabtagene vicleucel has also been approved for multiple myeloma patients after two or more prior therapies. Both CAR T-cell therapies improve progression-free survival compared to standard regimens in multiple myeloma patients.
References
[1] ASCO Post Staff (2024, April 8). Ciltacabtagene Autoleucel and Idecabtagene Vicleucel Approved by the FDA for Pretreated Patients With Multiple Myeloma. https://ascopost.com/news/april-2024/ciltacabtagene-autoleucel-and-idecabtagene-vicleucel-approved-by-the-fda-for-pretreated-patients-with-multiple-myeloma/
[2] Yu, B., Jiang, T., & Liu, D. (2020). BCMA-targeted immunotherapy for multiple myeloma. Journal of hematology & oncology, 13, 1-24.
[3] San-Miguel, J., Dhakal, B., Yong, K., Spencer, A., Anguille, S., Mateos, M. V., … & Einsele, H. (2023). Cilta-cel or standard care in lenalidomide-refractory multiple myeloma. New England journal of medicine, 389(4), 335-347.
[4] FDA ( 2022, March 30). FDA D.I.S.C.O. Burst Edition: FDA approval of CARVYKTI (ciltacabtagene autoleucel) for the treatment of adult patients with relapsed or refractory multiple myeloma after four or more prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-disco-burst-edition-fda-approval-carvykti-ciltacabtagene-autoleucel-treatment-adult-patients
[5] Usmani, S. Z., Martin, T., Berdeja, J. G., Jakubowiak, A., Agha, M., Cohen, A. D., … & Jagannath, S. (2022). MM-181 CARTITUDE-1: Two-Year Post Last Patient in (LPI) Results From the Phase 1b/2 Study of Ciltacabtagene Autoleucel (Cilta-Cel), a B-Cell Maturation Antigen (BCMA)-Directed Chimeric Antigen Receptor T (CAR-T) Cell Therapy, in Patients With Relapsed/Refractory Multiple Myeloma (RRMM). Clinical Lymphoma Myeloma and Leukemia, 22, S410-S411.
[6] Usmani, S. Z., Berdeja, J. G., Jakubowiak, A., Agha, M., Cohen, A. D., Madduri, D., … & Jagannath, S. (2021). UPDATED RESULTS FROM THE CARTITUDE-1 STUDY OF CILTACABTAGENE AUTOLEUCEL, A B-CELL MATURATION ANTIGEN–DIRECTED CHIMERIC ANTIGEN RECEPTOR T CELL THERAPY, IN RELAPSED/REFRACTORY MULTIPLE MYELOMA. Hematology, Transfusion and Cell Therapy, 43, S272.
[7] Bristol Myers Squibb (2024, 20 March). Bristol Myers Squibb’s Abecma (idecabtagene vicleucel) Becomes First CAR T Cell Therapy Approved in the European Union in Earlier Lines for Triple-Class Exposed Relapsed and Refractory Multiple Myeloma. https://news.bms.com/news/corporate-financial/2024/Bristol-Myers-Squibbs-Abecma-idecabtagene-vicleucel-Becomes-First-CAR-T-Cell-Therapy-Approved-in-the-European-Union-in-Earlier-Lines-for-Triple-Class-Exposed-Relapsed-and-Refractory-Multiple-Myeloma/default.aspx
[8] Delforge, M., Baz, R. C., Cavo, M., Callander, N. S., Ghobadi, A., Rodriguez-Otero, P., … & Berdeja, J. G. (2020). KarMMa-3: a phase 3 study of idecabtagene vicleucel (ide-cel, bb2121), a BCMA-directed CAR T cell therapy vs standard regimens in relapsed and refractory multiple myeloma. Blood, 136, 24-25.
[9] FDA (2021, 29 March). FDA approves idecabtagene vicleucel for multiple myeloma. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-idecabtagene-vicleucel-multiple-myeloma
[10] Bristol Myers Squibb (2021, 26 March). U.S. Food and Drug Administration Approves Bristol Myers Squibb’s and bluebird bio’s Abecma (idecabtagene vicleucel), the First Anti-BCMA CAR T Cell Therapy for Relapsed or Refractory Multiple Myeloma. https://news.bms.com/news/details/2021/U.S.-Food-and-Drug-Administration-Approves-Bristol-Myers-Squibbs-and-bluebird-bios-Abecma-idecabtagene-vicleucel-the-First-Anti-BCMA-CAR-T-Cell-Therapy-for-Relapsed-or-Refractory-Multiple-Myeloma/default.aspx
Content Source:肿瘤药闻
