BCMA CAR-T Approval, Efficacy Comparison of Four RRMM CAR-T Between China and the United States
BCMA CAR-T Approval, Efficacy Comparison of Four RRMM CAR-T Between China and the United States
The 50th Annual Meeting of the European Society for Blood and Marrow Transplantation (EBMT) was recently held in the United Kingdom. A study presented at the conference, titled “Matching-Adjusted Indirect Comparison (MAIC) Analysis of the Efficacy of BCMA CAR-T Products in Relapsed or Refractory Multiple Myeloma (RRMM),”1 revealed for the first time an indirect comparison of the efficacy of four BCMA CAR-T products, including the overall response rate (ORR), complete response (CR) rate, and minimal residual disease (MRD) negativity rate. The results showed that in terms of ORR, equecabtagene autoleucel (equ-cel), the world’s first fully human-derived CAR-T product approved for marketing in China in 2023, demonstrated a trend of superiority over ciltacabtagene autoleucel (cilta-cel) and idecabtagene autoleucel (ide-cel), which are marketed in the United States. Regarding the CR rate, equ-cel showed significant superiority over ciltacabtagene autoleucel (cilta-cel).
To date, four BCMA CAR-T products have been approved globally for the treatment of adult relapsed or refractory multiple myeloma (RRMM), but there have been no head-to-head studies comparing the efficacy of these products. The study accepted by the EBMT conference used a matching-adjusted indirect comparison (MAIC) method to compare the efficacy of equ-cel, cilta-cel, zevor-cel (idecabtagene vicleucel), and ide-cel (idecabtagene autoleucel) based on their registration clinical trials. This method weights individual patient data from the intervention of interest to match the aggregate data from the control intervention, allowing for efficacy comparisons within a population that is balanced at baseline after matching.
In this MAIC analysis, the primary data source for equ-cel was the FUMANBA-1 Phase Ib/II registration clinical study, an open-label, single-arm, multicenter study aimed at evaluating the efficacy and safety of equ-cel, the world’s first fully human-derived chimeric antigen receptor (CAR)-T cell injection (development code CT103A, English name Equecabtagene Autoleucel, equ-cel), in patients with relapsed or refractory multiple myeloma (RRMM) who had received at least three prior lines of therapy and had a short-term relapse. With a median follow-up time of 18.07 months, deep and durable responses were observed in 103 evaluable patients. In subjects without prior CAR-T therapy, the ORR reached 98.9%, the sCR/CR rate reached 82.4%, and the MRD negativity rate was 97.8%, with a median time to MRD negativity of only 15 days. The 12-month progression-free survival (PFS) rate was 85.5%, and 81.7% of patients maintained MRD negativity for more than 12 months.3
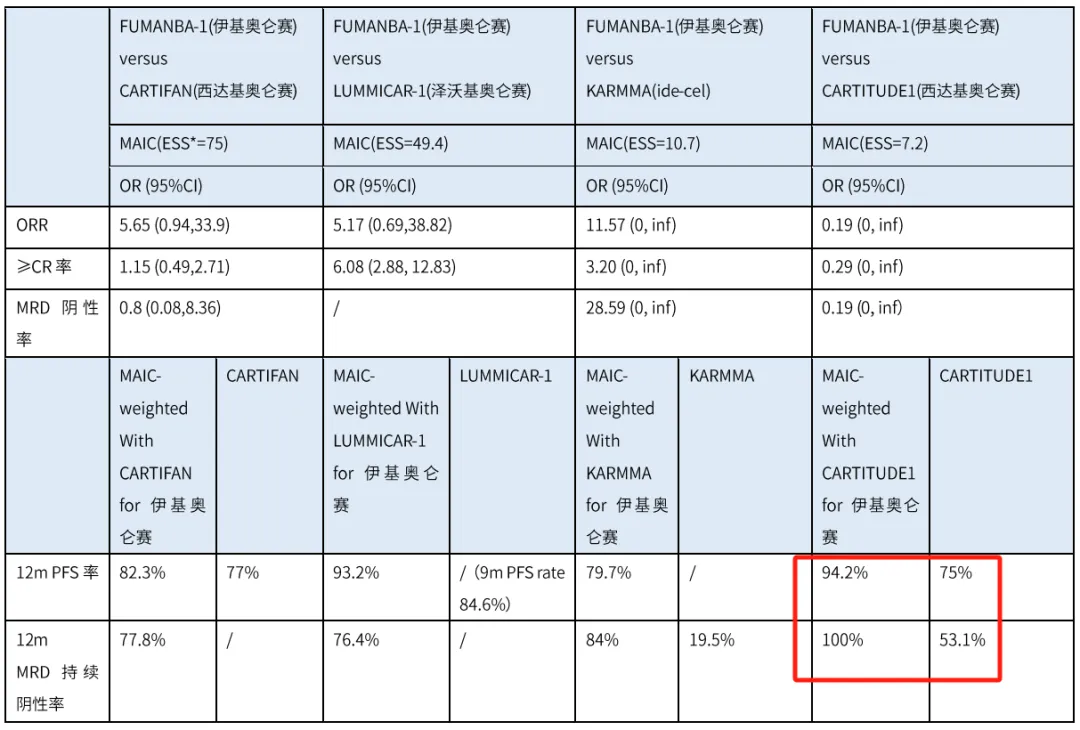
After weighting and matching the individual patient data from the FUMANBA-1 study in the MAIC analysis, in terms of ORR, equ-cel was 5.65 times higher than cilta-cel (CARTIFAN study), with an odds ratio (OR) of 5.65 and a 95% confidence interval (CI) of 0.94-33.9. For the CR rate, equ-cel was 6.08 times higher than zevor-cel (LUMMICAR study), with a statistically significant improvement advantage (OR = 6.08, 95% CI: 2.88-12.83).
Regarding long-term efficacy, the 12-month PFS rate for equ-cel was 94.2%, higher than the 75% reported for cilta-cel (CARTITUDE-1 study). Additionally, the 12-month sustained MRD negativity rate for equ-cel was 100%, higher than the 53.1% reported for cilta-cel (CARTITUDE-1 study). The comparisons of these two long-term efficacy indicators suggest that equ-cel, the BCMA CAR-T product approved in China, demonstrates a trend of superior long-term efficacy compared to cilta-cel, which is approved in the United States.
The data reported at this conference further demonstrate that equ-cel is a more promising treatment option for patients with relapsed or refractory multiple myeloma. After matching and adjusting for prognostic factors, and under conditions of relative baseline balance, equ-cel showed a trend of superior short-term and long-term efficacy improvement.
The principal investigators of this clinical study, Professor Qiu Lugui from the Blood Disease Hospital of the Chinese Academy of Medical Sciences and Professor Li Chunrui from Tongji Hospital affiliated with Tongji Medical College of Huazhong University of Science and Technology, stated: “BCMA CAR-T has already shown breakthrough efficacy compared to traditional treatments. In this MAIC analysis, which compares the efficacy of different CAR-T products under baseline-balanced conditions, equ-cel demonstrated superior short-term and long-term efficacy among the four BCMA CAR-T products in terms of important prognostic endpoints for MM patients, such as objective response rate, CR rate, MRD negativity rate, and sustained negativity rate. Furthermore, compared to the same class of drugs approved in the United States, equ-cel showed superior long-term efficacy. Different CAR-T products have different CAR structures and development approaches. The CAR structure of equ-cel was selected from numerous fully human candidate antibodies, with the fully human antibody light and heavy chains binding tightly to the BCMA antigen, enhancing the cytotoxic ability (Figure 1). The low immunogenicity allows equ-cel to persist longer in the patient’s body. Additionally, the dissociation constant (Kd) of equ-cel is very close to the natural dissociation kinetics of human T cells2 (Figure 2): rapid dissociation facilitates efficient activation, killing, and proliferation of CAR-T cells in the body, enhancing cytotoxic efficiency and response depth while reducing self-exhaustion of equ-cel, allowing it to persist for an extended period in the patient’s body and maintain long-term surveillance, reducing the risk of relapse.”
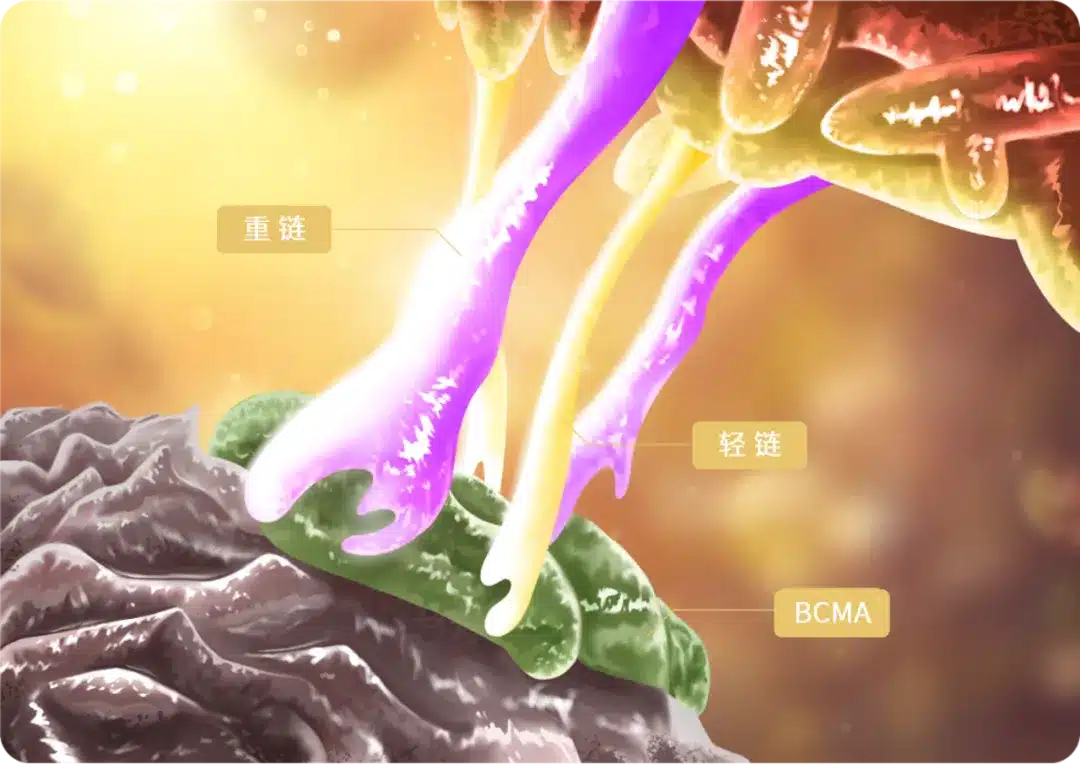
Figure 1
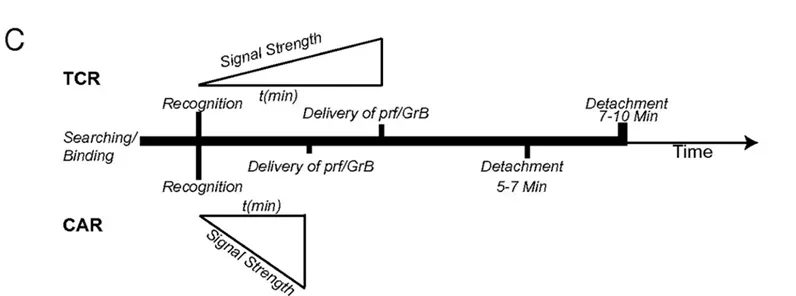
Figure 2: The ideal dissociation time for human T cells attacking tumor cells is 5-7 minutes.
In the registration clinical trial involving the Chinese population, equ-cel also demonstrated excellent safety: ≥Grade 3 CRS was only 1%; no ≥Grade 3 ICANS; no movement/cognitive impairment, no Parkinson’s disease, and no secondary tumors were observed.3 With 10 months of clinical application experience in China, this will help deepen the understanding of the efficacy and safety of BCMA CAR-T therapy in the Chinese population.
Currently, BCMA CAR-T products are primarily approved for the treatment of relapsed or refractory multiple myeloma, but their potential application in other indications, such as severe autoimmune diseases like myasthenia gravis, has also made promising progress in China. The domestically developed and fully produced BCMA CAR-T product in China has demonstrated outstanding efficacy and safety, with broad global application prospects, bringing hope for a cure to more Chinese and international patients.
Reference Materials:
[1] 2024 EBMT, Abstract A092.
[2] Davenport, A. J, et al. Proceedings of the National Academy of Sciences 115, no. 9: E2068–76.
[3] 2023 IMS. P-290.
