
CAR-T
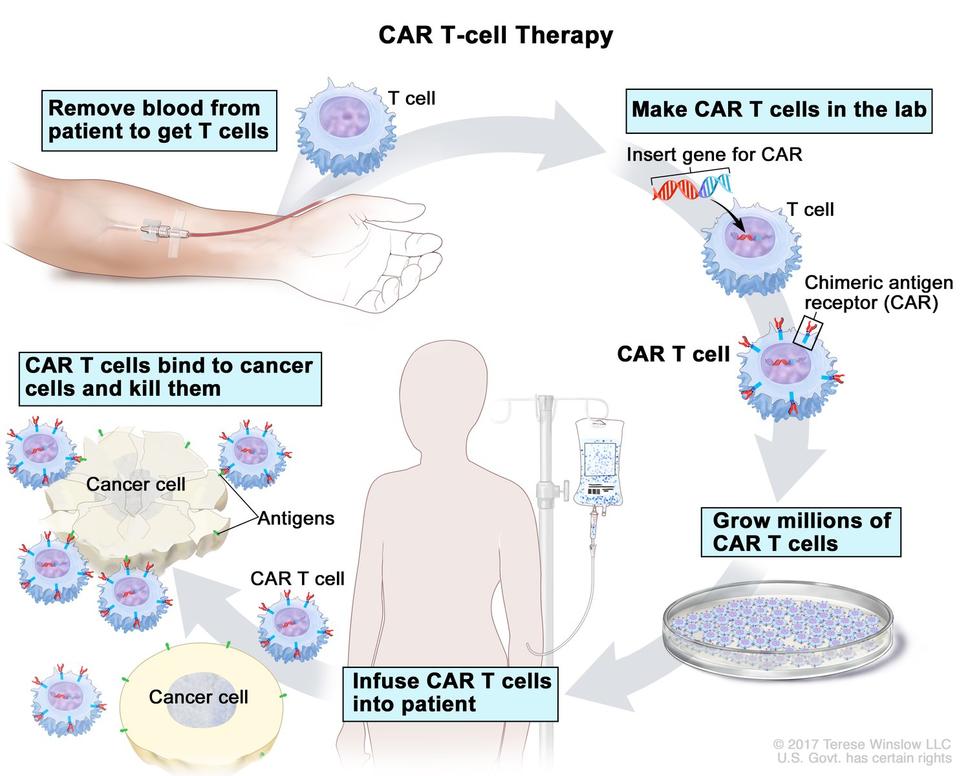
Immunotherapy enhances the power of a patient’s immune system to attack tumors. An immunotherapy approach, called chimeric antigen receptor (CAR) T-cell therapy, uses patients’ own immune cells to treat their cancer
What Is CAR T Cell Therapy?
CAR T cell therapy provides engineered molecules called chimeric antigen receptors (CARs) that recognize and destroy antigens present on the surface of lymphoma cells. T cells are removed from patients and genetically modified to produce CARs. The genetically engineered CAR T cells are grown in the laboratory until they number in the billions and are then infused back into the patient.

What is Lymphoma?
Chronic Lymphocytic Leukemia ›
Hodgkin Lymphoma ›
Non-Hodgkin Lymphoma ›
Treatment Options ›

What resources are available?
Fact Sheets and Guides ›
Education Programs ›
Helpline ›
Lymphoma Support Network ›

What can I do to help?
Get Involved ›
Find a Walk or Endurance Event ›
Create a Fundraiser ›
Submit a Story of Hope ›
How Does CAR T-cell Therapy Work?
In CAR T-cell therapy for lymphoma, a lab modifies a patient's blood cells to help them fight cancer cells in the blood. Once a patient's blood is drawn, a lab engineers the T-cells to gain a chimeric antigen receptor (CAR), which can bind to a protein on the cancer cells. The CAR T-cells are multiplied to produce more engineered T-cells. When there are enough of these special cells, they are reintroduced to the patient via a blood infusion.malignant plasma cells then produce an abnormal antibody called M protein, high levels of which are a hallmark characteristic of multiple myeloma.
Approved CAR T Cell Therapies in Lymphoma
Approved CAR T Cell therapies include:
| Approved drug | Approved drug indication |
|---|---|
| Axicabtagene Ciloleucel (Yescarta) |
|
| Brexucabtagene Autoleucel (Tecartus) | |
| Lisocabtagene Maraleucel (Breyanzi) |
|
| Tisagenlecleucel (Kymriah) |
|
CAR T Cell Process
1. Leukapheresis
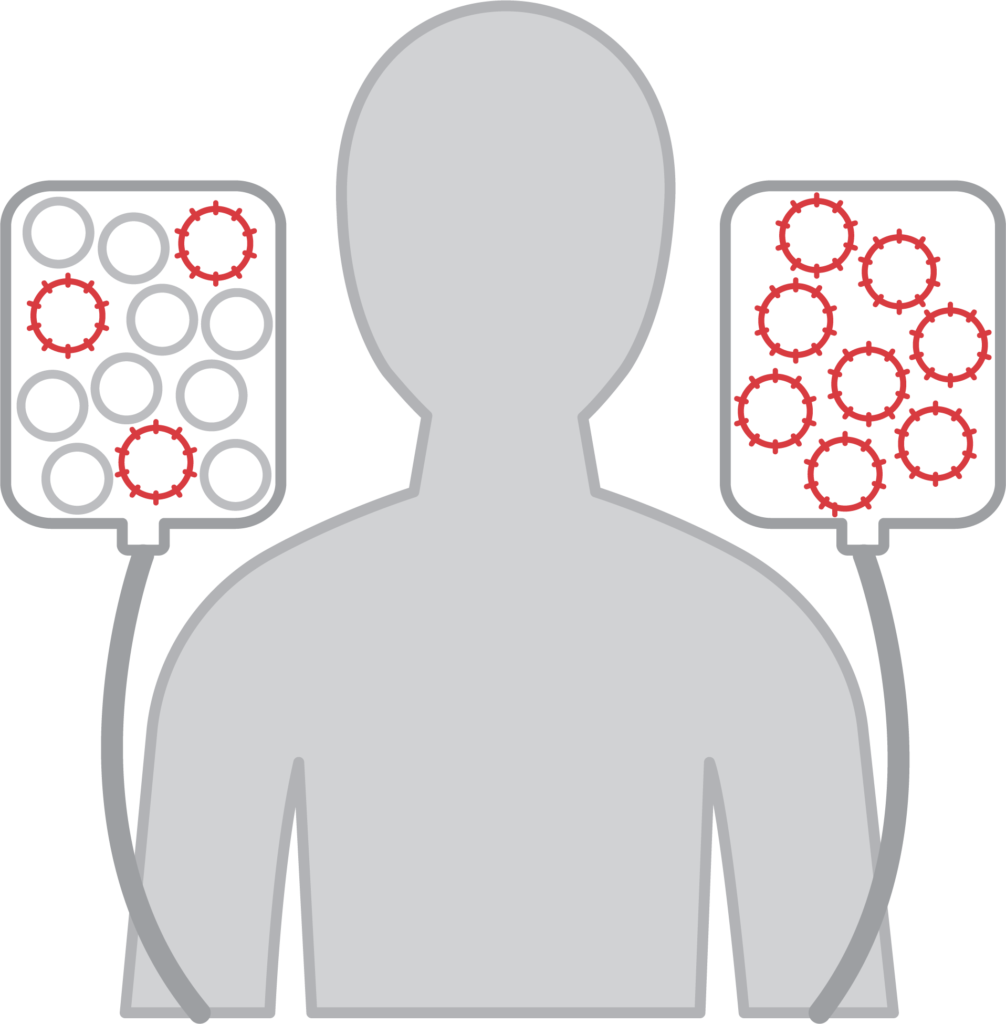
Your T cells are obtained through a process called leukapheresis, which usually takes three to four hours.
2. T-Cell Engineering
The T cells are sent to a processing center where they are genetically engineered to target your lymphoma.
3. CAR T Cell Transport
Once enough of the CAR-T cells are available at the processing center, the cells are frozen for transport to your certified treatment center.
4. Lymphodepleting Chemotherapy
A few days prior to your CAR-T cell infusion, you will receive low-dose chemotherapy.
5. CAR T Cell Infusion
A few days after completing chemotherapy, you will receive your CAR-T cells at your certified treatment center.
6. CAR T Cells Attack the Lymphoma
Once the CAR-T cells enter your body, they begin to multiply and attack the lymphoma cells.
Patient Stories
-
**Иностранные пациенты ищут лечение: Лучшая терапия CAR-T в Китае! Первый российский пациент с множественной миеломой успешно очистил опухоль и достиг CR!** В течение пяти лет после постановки диагноза высокорисковой множественной миеломы, господин Ф. испытывал невыносимые страдания. Несмотря на различные схемы химиотерапии и два пересадок, ему не удавалось полностью избавиться от кошмара непредсказуемых рецидивов и прогрессирования Read More
-
**Seeking Medical Treatment Abroad: The Best CAR-T Therapy is in China! The First Russian Multiple Myeloma Patient Successfully Achieves Complete Tumor Clearance and CR!** Over the five years following his diagnosis of high-risk multiple myeloma, Mr. F endured relentless pain. Despite undergoing various chemotherapy regimens and two transplants, he could never completely escape the nightmare Read More











