Multiple Myeloma Therapy China, Bispecific BCMA/CD19 CAR-T Cell Therapy Opens New Era in the Treatment of Relapsed/Refractory Multiple Myeloma
Multiple Myeloma Therapy China, Bispecific BCMA/CD19 CAR-T Cell Therapy Opens New Era in the Treatment of Relapsed/Refractory Multiple Myeloma
Cao Jiang, Xu Kailin, professors from the Department of Hematology, Xuzhou Medical University Affiliated Hospital, and Zheng Junnian, Shi Ming, professors from the Cell Therapy Drug Industry College, Xuzhou Medical University, China, conducted a phase I/II clinical trial to evaluate the efficacy and safety of CAR-T cell therapy targeting BCMA and CD19 in patients with relapsed/refractory multiple myeloma (R/R MM). On April 20, 2024, the results of this trial were published online in Nature Communications. Let’s take a closer look at the details.
Research Background
Multiple myeloma (MM) is the second most common hematological malignancy, characterized by clonal proliferation of plasma cells in the bone marrow. MM is currently incurable, and treatment options for relapsed and refractory (R/R) MM patients remain suboptimal. However, BCMA-targeted CAR-T cell therapy has shown an overall response rate (ORR) of 48%-100% in heavily pretreated and refractory MM patients. Nevertheless, 4%-33% of patients exhibit downregulation or loss of BCMA expression, prompting the consideration of designing novel bispecific CAR-T cell therapies, drawing from the experience of CD19/CD22-targeted CAR-T cell therapy for leukemia and lymphoma, CD19/CD20-targeted CAR-T cell therapy for lymphoma, and BCMA/CD38-targeted CAR-T cell therapy for MM.
Previous studies have shown that the expression of CD19 on some MM cells is associated with drug resistance and poor survival. The administration of anti-CD19 CAR-T cells after high-dose melphalan and salvage autologous hematopoietic stem cell transplantation (auto-HSCT) has also demonstrated the potential role of CD19 in R/R MM patients. Driven by these findings, a previous prospective study demonstrated the feasibility of co-infusing humanized anti-CD19 CAR-T cells and anti-BCMA CAR-T cells in R/R MM patients.
Based on these data, a second-generation bispecific BC19 CAR targeting BCMA and CD19 was designed. This trial reports the preclinical results of BC19 CAR-T cells and the outcomes of R/R MM patients treated in the phase I/II trial.
Research Methods
This open-label, single-arm, multicenter phase I/II trial enrolled R/R MM patients aged <70 years, with an ECOG performance status ≤2, who had relapsed or previously received at least two lines of ineffective treatment. Patients received BC19 CAR-T cell infusion (1×10^6/kg) on day 0, followed by lymphodepleting cyclophosphamide (750 mg/m^2) on day 5, and fludarabine (30 mg/m^2) from days 2 to 5. Efficacy assessments were performed on days 14 and 28 after infusion, and at each follow-up visit.
The primary endpoint was safety, and key secondary endpoints included overall response rate (ORR), stringent complete response (sCR) rate, complete response (CR) rate, very good partial response (VGPR) rate, or partial response (PR) rate. Other secondary endpoints included duration of response (DOR), progression-free survival (PFS), and overall survival (OS).
Research Results
Patient Characteristics
The study enrolled 64 eligible patients, and 50 patients ultimately received BC19 CAR-T cell infusion. The median age of patients was 57 years (range, 31-70), and the median time from diagnosis to treatment was 29.5 months (range, 4-162). Forty-six patients (96%) were in stage II or III, 7 patients (14%) had extramedullary disease, and 34 patients (68%) had high-risk cytogenetic features. The median number of prior treatment lines was 4 (range, 2-11), with 20 patients (40%) having previously received auto-HSCT and 5 patients (10%) having previously received BCMA, CD19, or GPRC5D-targeted CAR-T cell therapy. Two patients received auto-HSCT consolidation at 2.3 and 3.8 months after CAR-T cell therapy, respectively.
Safety
The most common adverse events (AEs) after CAR-T cell infusion were hematological AEs and cytokine release syndrome (CRS). All 50 patients experienced hematological AEs, including neutropenia (100%), leukopenia (100%), anemia (94%), and thrombocytopenia (88%). The most common grade 3-4 hematological AEs were neutropenia (98%), leukopenia (96%), thrombocytopenia (66%), and anemia (64%). Patients with grade 3-4 cytopenias on day 0 after BC19 CAR-T cell infusion recovered to ≤ grade 2 AEs by day 28. Forty-six patients (92%) experienced CRS, with 4 patients (8%) experiencing ≥ grade 3 CRS.
Forty-two patients (84%) received granulocyte colony-stimulating factor (G-CSF) for the treatment of neutropenia after CAR-T therapy. Within the first 3 months after CAR-T cell infusion, 30 patients (60%) experienced infections, with 66.7% being mild to moderate. Among the 49 patients who survived beyond 3 months, 19 patients (39%) had infections lasting beyond 3 months, with 10 patients (20%) experiencing severe infections. Except for one patient with a grade 5 infection, all infections were appropriately and timely treated. At the time of data cutoff, 8 patients (16%) had died, with 4 patients (8%) dying due to disease progression or related complications.
Efficacy
Among the 50 evaluable patients for efficacy, the ORR was 92% (95% CI: 81-98), with an sCR rate of 36%, a CR rate of 24%, a VGPR rate of 18%, and a PR rate of 16%. Eight percent of patients achieved stable disease (SD). The median time to first VGPR was 23.5 days (range, 14-30), and the median time to best response was 1.9 months (range, 0.5-6.0). Six out of 7 patients (86%) with extramedullary disease achieved an overall response, and among the 5 patients previously treated with CAR-T cell therapy, 2 achieved sCR, 1 achieved PR, and 2 achieved SD.
With a median follow-up of 11 months (range, 2.3-24), the median OS and median PFS were 19.7 months (95% CI: 5.0-34.4) and 19.7 months (95% CI: 7.7-31.7), respectively, and the median DOR was not reached (Figure 1). For patients achieving VGPR, the 1-year PFS rate, 1-year OS rate, and 1-year DOR rate were 55% (95% CI: 38-69), 85% (95% CI: 71-93), and 59% (95% CI: 41-73), respectively. For patients achieving CR or better, the 1-year PFS rate and 1-year OS rate were 66% (95% CI: 45-81) and 90% (95% CI: 72-97), respectively.
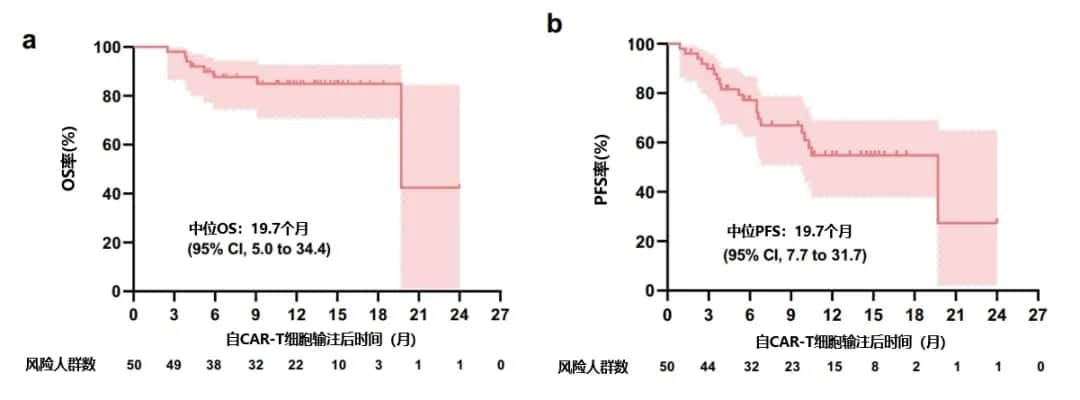
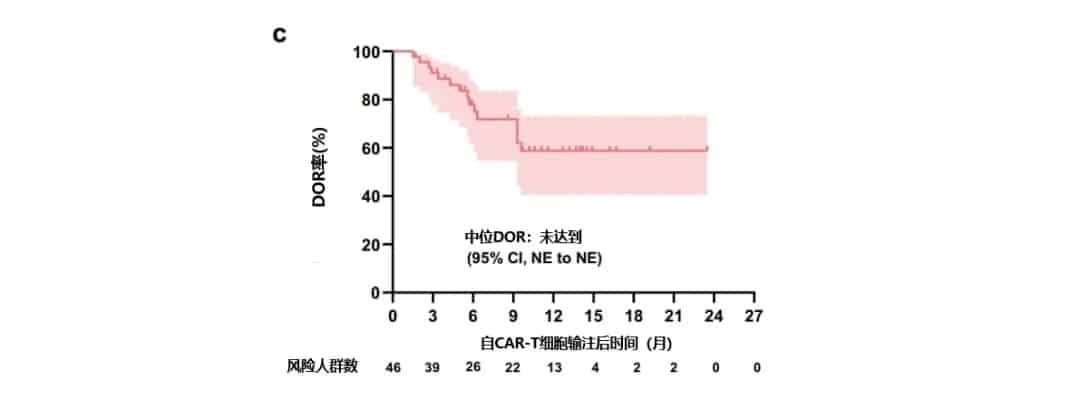
Figure 1. Patient OS (a), PFS (b), and DOR (c)
Expansion and Persistence
The peripheral blood expansion of CAR-BC19 peaked at day 12 after infusion and then gradually declined, with detection rates of 86%, 62%, 44%, and 28% at 3, 6, 9, and 12 months, respectively. Post hoc analysis revealed that within the first 28 days, responders had significantly higher peak and cumulative levels of CAR-BC19 transgene compared to non-responders.
Research Conclusion
The preliminary results of this phase I/II trial demonstrated that bispecific BC19 CAR-T cell therapy exhibited promising response rates, depth of response, and durability in R/R MM patients, with an ORR of 92%, a CR and sCR rate of 62%, and a 1-year OS rate of 85%, showing significant antitumor activity compared to other regimens. However, larger sample sizes and longer follow-up periods in future prospective and multicenter clinical trials are needed to explore the impact of BC19 CAR-T cell therapy on long-term outcomes.
References
Shi M, Wang J, Huang H, et al. Bispecific CAR T cell therapy targeting BCMA and CD19 in relapsed/refractory multiple myeloma: a phase I/II trial. Nat Commun. 2024 Apr 20;15(1):3371. doi: 10.1038/s41467-024-47801-8.
Content Source:聚焦CA
