The Hope of CAR-T Cell DLBCL Cure
The Hope of CAR-T Cell DLBCL Cure
A Case of CAR-T Cell Therapy for Relapsed/Refractory Diffuse Large B-Cell Lymphoma
Can a single injection eliminate cancer cells? If it weren’t for her own personal experience, Ms. Zhou, who had been tormented by relapsed/refractory diffuse large B-cell lymphoma (DLBCL) for a long time, would hardly believe it.
Ms. Zhou is 53 years old this year. In February 2023, she was admitted to the Department of Lymphoma, Blood and Pediatric Oncology, Affiliated Tumor Hospital of Guangxi Medical University due to a relapse of DLBCL. Previously, Ms. Zhou had received autologous hematopoietic stem cell transplantation, but with poor results.
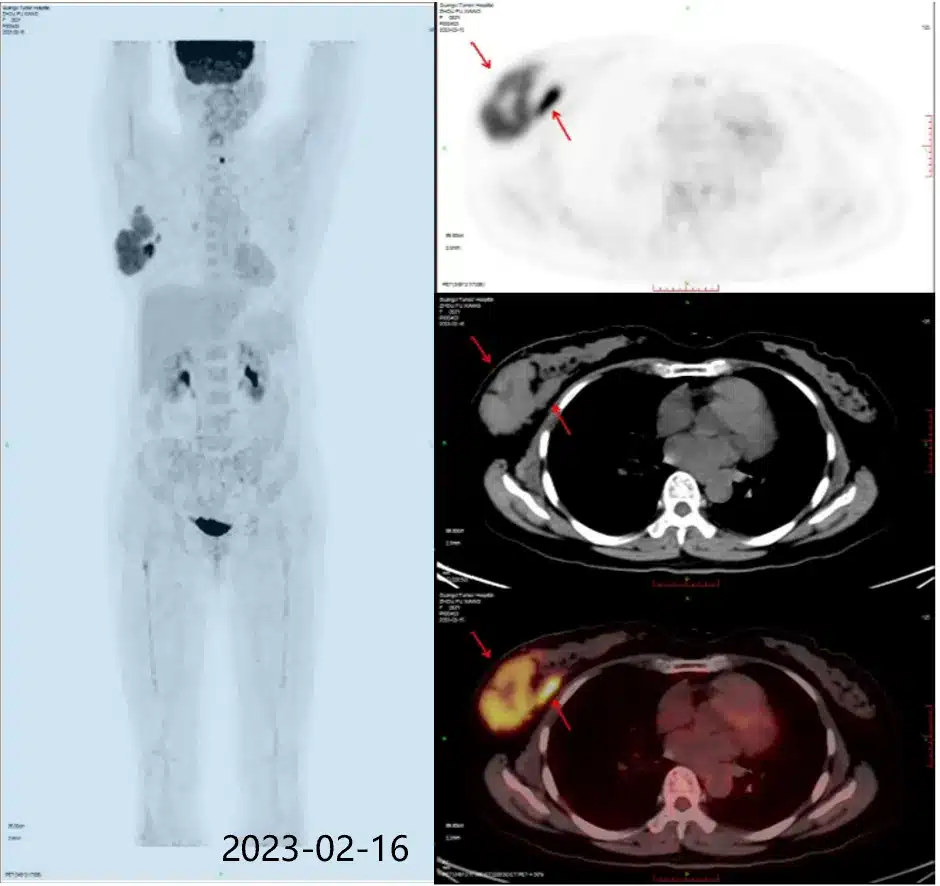
△ Ms. Zhou’s pre-treatment PET-CT result
In response to Ms. Zhou’s condition, Dr. Cen Hong, the director, and Dr. Tan Xiaoqing, the associate director of the Department of Lymphoma, Blood and Pediatric Oncology, organized a multidisciplinary team (MDT) consultation involving experts from the departments of radiotherapy, pathology, medical imaging center, and others. Based on the latest developments in the treatment of DLBCL at home and abroad, they thoroughly discussed and concluded that for a difficult-to-treat case like Ms. Zhou, who relapsed after transplantation, the likelihood of traditional treatment being effective was extremely low. The most effective treatment that could give her a chance for long-term survival was chimeric antigen receptor T-cell (CAR-T) therapy.

△ Pre-treatment MDT consultation organized by Dr. Cen Hong
What is CAR-T Cell Therapy
CAR-T cell therapy is a major breakthrough in lymphoma treatment in recent years and is a form of immunotherapy. The principle of CAR-T therapy is to use genetic engineering techniques to activate the body’s immune “warrior” T cells and install a positioning and navigation device called CAR (chimeric antigen receptor). This transforms ordinary “soldier” T cells into “super soldiers,” known as “CAR-T cells.” They can release various effector factors through immune actions, effectively killing tumor cells, and thus achieving the treatment of malignant tumors.
CAR-T cell therapy mainly involves three steps: first, collecting the patient’s T cells; second, modifying the T cells to become CAR-T cells; and third, infusing the CAR-T cells back into the patient’s body to kill the cancer cells.
After thorough communication with the experts, the patient, and her family, the decision was made to proceed with CAR-T cell therapy for Ms. Zhou. On February 18, 2023, the medical staff successfully collected a sufficient quantity of lymphocytes from Ms. Zhou. After the CAR-T cells were prepared, they were infused back into her on March 22nd. She was discharged after safely passing through the post-infusion adverse reaction period.

△ Associate Director Tan Xiaoqing and the medical team conducting rounds after infusion
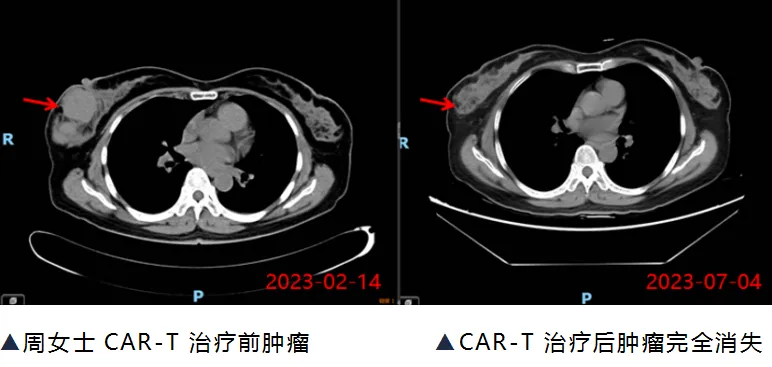
Three months later, Ms. Zhou returned for a follow-up CT scan, which showed that the tumor had completely disappeared, and her circulating tumor DNA (ct-DNA) result was negative. Ms. Zhou became the first patient in Guangxi to receive commercial CAR-T cell therapy and be successfully cured.

△ Ms. Zhou was discharged after recovery
Following Ms. Zhou’s case, CAR-T cell therapy is bringing hope to more patients with relapsed/refractory DLBCL. Mr. Huang, aged 58, and Ms. Li, aged 48, also received CAR-T cell therapy at our Department of Lymphoma, Blood and Pediatric Oncology. They were both DLBCL patients who had failed traditional treatments. After the CAR-T cell therapy, follow-up examinations showed significant tumor shrinkage, and they are currently under close observation, welcoming a new dawn of life from the brink of despair.
About Lymphoma and CAR-T Cell Therapy
Lymphoma is a type of malignant tumor that primarily occurs in the lymphatic system. Lymphomas are divided into two main categories: Hodgkin lymphoma and non-Hodgkin lymphoma (NHL), with NHL accounting for the vast majority, approximately 85%. Among NHLs, diffuse large B-cell lymphoma (DLBCL) is the most common, accounting for about 40%. DLBCL is a highly aggressive tumor, and if left untreated, patients have a very short survival period. Currently, the standard first-line treatment is the RCHOP regimen, which includes rituximab (an anti-CD20 monoclonal antibody) combined with cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy. This regimen can cure approximately 60% of patients. However, 40% of patients will still relapse or become refractory (R/R), and for these R/R DLBCL patients, CAR-T cell therapy can bring them hope for a new lease on life.
Characteristics of CAR-T Cell Therapy
Highly individualized: Each patient’s CAR-T cells are customized according to their specific situation, eliminating the risk of rejection or infection.
Persistent: CAR-T cells can survive and proliferate in the body for an extended period, forming memory immune cells that continuously monitor and eliminate recurring tumors.
Highly effective: CAR-T cells can penetrate blood vessel walls and tumor stroma, directly contacting and killing tumor cells while releasing a large number of cytokines, activating other immune cells to participate in tumor elimination.
Efficacy of CAR-T Cell Therapy
Multiple clinical trials have demonstrated the significant efficacy of CAR-T cell therapy for R/R DLBCL patients. Among them, the ZUMA-1 and ZUMA-7 studies are the most representative.
The ZUMA-1 study was a multicenter, single-arm, open-label phase I/II clinical trial involving R/R DLBCL patients. A total of 101 patients were enrolled, and 91 successfully received CAR-T cell therapy. The results showed that one month after treatment, the objective response rate (ORR) was 83%, and the complete response rate (CR) was 58%. Two years after treatment, the ORR was 39%, and the CR was 36%. This means that nearly a quarter of patients achieved long-term disease-free survival after receiving CAR-T cell therapy.
The ZUMA-7 study was a multicenter, randomized, open-label phase III clinical trial involving R/R DLBCL patients. A total of 359 patients were enrolled, with 235 receiving CAR-T cell therapy and 124 receiving standard second-line chemotherapy. The results showed that one month after treatment, the ORR was 65%, and the CR was 37%. Two years after treatment, the ORR was 41%, and the CR was 32%. Compared to standard chemotherapy, CAR-T cell therapy significantly improved patients’ progression-free survival (PFS) and overall survival (OS) while reducing the risk of death.
Adverse Reactions of CAR-T Cell Therapy
Although CAR-T cell therapy has shown significant efficacy for R/R DLBCL patients, it is not without risks. The most common adverse reactions of CAR-T cell therapy are cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS). Both of these reactions are caused by the massive release of cytokines by CAR-T cells in the body, resulting in systemic inflammatory responses characterized by fever, hypotension, respiratory distress, and neurological abnormalities. Generally, these reactions can be alleviated or controlled by administering drugs such as tocilizumab (an interleukin-6 inhibitor) and dexamethasone (an immunosuppressant). However, severe reactions can be life-threatening.
Therefore, before proceeding with CAR-T cell therapy, doctors will develop an individualized treatment plan based on the patient’s specific condition, including bridging chemotherapy, preconditioning regimen, dose selection, and infusion timing. During the treatment process, doctors will closely monitor the patient’s vital signs and laboratory indicators to promptly detect and manage adverse reactions. In general, most patients can tolerate and safely complete CAR-T cell therapy.
Content Source:广西医科大学附属肿瘤医院
