How Effective is CAR-T Kymriah (Tisagenlecleucel) in Treating Relapsed or Refractory Follicular Lymphoma
How Effective is CAR-T Kymriah (Tisagenlecleucel) in Treating Relapsed or Refractory Follicular Lymphoma
Kymriah (Tisagenlecleucel) is an autologous anti-CD19 chimeric antigen receptor (CAR) T-cell therapy that has shown clinically meaningful results in patients with relapsed/refractory B-cell lymphoma. The FDA approved the first CAR-T cell therapy, authorizing the use of Kymriah for the treatment of patients up to 25 years of age with B-cell precursor acute lymphoblastic leukemia (ALL). In preliminary studies for the treatment of relapsed/refractory follicular lymphoma (FL) with Kymriah, 71% of patients achieved a complete response (CR).
In the past two years, a research article published in the prestigious medical journal Nature Medicine reported the preliminary, prespecified interim analysis of the multinational phase 2 ELARA trial for Tisagenlecleucel. This trial was conducted in adult patients with relapsed/refractory FL (NCT03568461) who had received two or more prior lines of therapy or required autologous stem cell transplantation. The primary endpoint was the CR rate (CRR), while secondary endpoints included overall response rate (ORR), duration of response, progression-free survival, overall survival, pharmacokinetics, and safety.

As of March 29, 2021, 97/98 patients enrolled in the study received Kymriah treatment (median follow-up of 16.59 months; IQR, 13.8-20.21), and the study’s primary endpoint was met. In the efficacy population (n=94), the CRR was 69.1% (95% CI, 58.8-78.3), and the ORR was 86.2% (95% CI, 77.5-92.4).
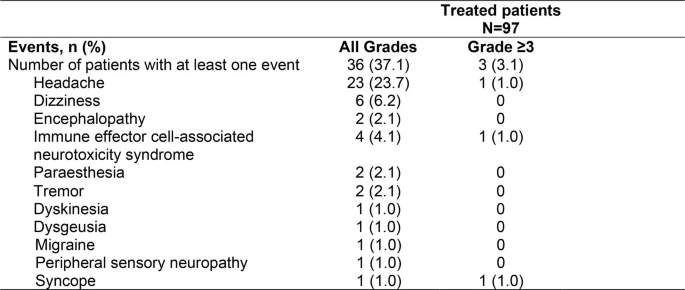
In the safety population (n=97), the incidence of cytokine release syndrome within 8 weeks of Kymriah infusion was 48.5% (Grade ≥3, 0%), neurological events were 37.1% (Grade ≥3, 3%), and immune effector cell-associated neurotoxicity syndrome (ICANS) was 4.1% (Grade ≥3, 1%). No treatment-related deaths occurred.
Thus, Kymriah demonstrated a favorable safety and efficacy profile in heavily pretreated patients with relapsed/refractory FL, including high-risk patients.
