What is the Efficacy of Diffuse Large B-cell Lymphoma (DLBCL) CAR-T Treatment
What is the Efficacy of Diffuse Large B-cell Lymphoma (DLBCL) CAR-T Treatment
Diffuse large B-cell lymphoma (DLBCL) is a common aggressive hematological malignancy, with approximately 60% of patients achieving complete remission (CR) after treatment with anthracycline-containing regimens and rituximab. However, in patients with relapsed/refractory DLBCL (r/rDLBCL), the percentage of patients with a 1-year survival rate is less than 30%. Although autologous anti-CD19 CAR-T cell therapy is highly effective in treating large B-cell lymphomas, less than half of r/rDLBCL patients experience a response duration exceeding 1 year.
Loss or mutation of CD19 antigen expression is a common mechanism of CAR-T cell resistance and relapse in B-cell malignancies. However, not all relapsed patients present with antigen-negative disease, indicating that other factors may contribute to resistance. Therefore, identifying the factors leading to treatment resistance and how to overcome these factors through innovative CAR-T cell design has been a major focus of research in this field in recent years.

In October 2023, Yao Wang and colleagues from the Department of Biotherapy at the First Medical Center of the Chinese PLA General Hospital published a research article titled “Characteristics of premanufacture CD8+ T cells determine CAR-T efficacy in patients with diffuse large B-cell lymphoma” in the journal Signal Transduction and Targeted Therapy. The authors conducted a follow-up study on DLBCL patients who received CD19/CD20 tandem CAR-T cell (coded TanCAR7 T) therapy. They analyzed the in vitro cytotoxicity of the infused CAR-T cell products against CD19/CD20-positive tumor cells from 56 evaluable patients, comparing the anti-tumor functions of CAR-T cells from resistant patients with those from patients with durable responses (DR). They analyzed the correlations between the composition, functions, and gene expression profiles of the CAR-T cell products and premanufacture cells with clinical efficacy to identify features associated with sustained clinical responses. Finally, the authors proposed that the quantity and function of CD8+ stem cell memory T cells (TSCM) in the CAR-T cell products are crucial factors for achieving long-term remission, while the absence of initial CD8+ naive T cells may lead to CAR-T cell therapy resistance.
CAR-T cells from responding patients exhibit superior anti-tumor and activation capabilities compared to those from resistant patients
The authors first analyzed the relationship between individual differences, tumor heterogeneity, and CAR-T cell efficacy, finding that the number of prior treatment lines, previous treatment responses, tumor Ki67 expression, and gene mutations were independent of the treatment outcome. They then examined the ability of clinically infused CAR-T cell products to kill human leukemia cells over time to assess their anti-tumor efficacy. The results showed that CAR-T cells from CR patients more effectively killed Nalm6 tumor cells and secreted higher levels of TNFα and IFNγ (Figure 1) with increased CD107a expression compared to those from patients with progressive disease (i.e., resistant patients).
The authors then investigated the specific TCR or CAR functional activity upon CAR-T cell activation. When stimulated with either a downstream TCR signal inducer (ionomycin) or Nalm6 cells, the CD8+CD25+ CAR-T cell population from CR patients expressed higher levels of granzyme B (GZMB), IFNγ, and TNFα compared to those from resistant patients. These data indicate that prior to cell therapy infusion, CAR-T cells from responding patients exhibited superior anti-tumor and activation capabilities compared to those from resistant patients.
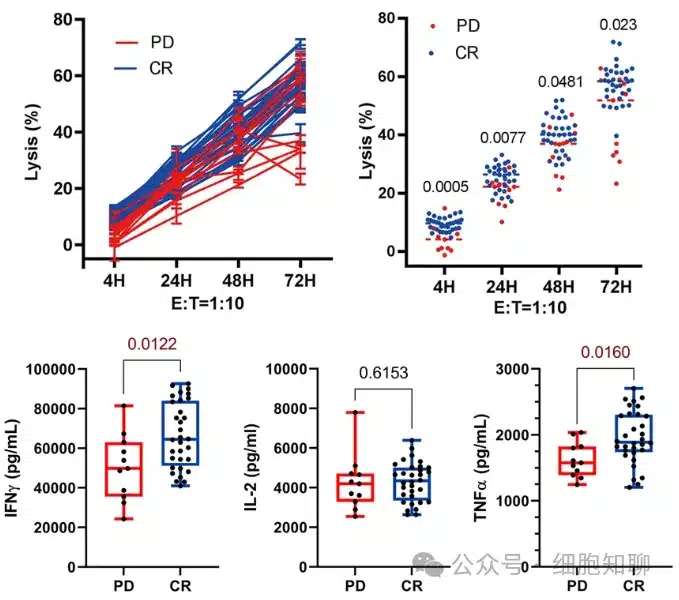
▲ Figure 1: CAR-T cells obtained from CR patients exhibit stronger tumor-killing ability and higher TNFα and IFNγ secretion.
CD8+ TSCM CAR-T cells play a crucial role in the response to CAR-T cell therapy
In vivo expansion of CAR-T cells in responding patients was primarily driven by CD8+ T cells, typically starting 5 days after cell infusion and lasting 3-4 weeks. Among the CAR-T cell products from all 56 evaluable DLBCL patients, the proportion of CD8+ stem cell memory T cells (TSCM; CD45RA+CD62L+) was significantly higher in responding patients than in resistant patients (p<0.0001), with patients in the highest quartile showing a 100% response rate. When stratified into high and low CD8+ TSCM level groups based on the CAR-T cell products, patients with higher CD8+ TSCM levels exhibited slightly better progression-free survival (PFS); among patients with high tumor burden, those with higher CD8+ TSCM content had longer PFS (Figure 2). These data indicate that the quantity of CD8+ TSCM cells in the CAR-T cell products significantly distinguished between patients with durable remission and those with relapsed or resistant disease. A lack of CD8+ TSCM cells in the infused CAR-T cells may be a major contributing factor to primary treatment resistance.
To determine whether the sustained response to CAR-T cell therapy was a result of the numerical advantage of CD8+ CAR-T cells, the authors performed bulk RNA-seq analysis on CD8+ CAR-T cell products from durable responders (DR) and resistant patients. The results showed differential gene expression patterns between the CD8+ CAR-T cell products from responding and resistant patients. Most memory-associated genes, such as CD27, TCF7, CCR7, LEF1, BACH2, SELL, and CAPG, were significantly upregulated in CD8+ CAR-T cells from responding patients, while activation and exhaustion-related genes, including GZMK, TBX21, EOMES, TOX, TIGIT, and SP140, were significantly downregulated. Gene set enrichment analysis (GSEA) revealed that memory-associated and quiescence-associated genes were enriched in CD8+ CAR-T cells from responding patients compared to those from resistant patients, indicating a relatively quiescent state.
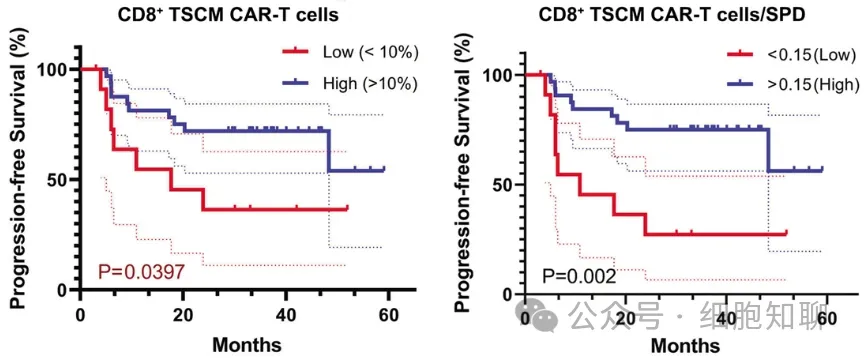
▲ Figure 2: Patients with higher CD8+ TSCM population levels exhibit longer PFS.
Lack of CCR7 expression and increased expression of activation/inhibitory signature genes in infused CD8+ CAR-T cells are associated with CAR-T cell therapy resistance
To comprehensively characterize the efficacy-associated CAR-T cell populations, the research team performed 5′ scRNA-seq and paired T-cell receptor sequencing analysis on CAR-T cell products from 3 responding and 3 resistant patients (Figure 3). Through data clustering, they identified 21 clusters with distinct transcriptomic features and defined different clusters based on cell state-associated genes or function-related genes.
The results revealed heterogeneity in T-cell activation, cell proliferation, immune response, AP-1 complex, cell adhesion, lysosome, and IFN-γ response across patient CAR-T cell products. Cells in clusters C2, C6, C9-10, and C16-18 expressed the highest levels of resistant patient-associated genes (over 80%), including some exhausted/terminal CD8+ T cells expressing LAG3, TIGIT, GZMB, GZMK, GZMA, GZMH, NKG7, and EOMES. Further analysis of these effector clusters lacking memory-associated gene expression revealed significantly higher average expression levels of T-cell activation-related transcription factors TBX21, EOMES, and NKG7, along with increased inhibitory factor expression, including CD38, LAG3, TIGIT, ADA, and ID2, in CAR-T cells from resistant patients compared to those from responding patients.
TSCM cells are a relatively small subgroup of memory lymphocytes characterized by high expression of stem cell memory-like properties, including TCF7, LEF1, SELL, CCR7, CD27, IL7R, and TNFRSF6. In responding patients, the expression levels of LEF1, CCR7, and TCF7 in CAR-T cells were higher than in resistant patients, and cells expressing CCR7 were significantly increased in CD8+ CAR-T cells. These results suggest that the decreased expression of CCR7 in CD8+ memory CAR-T cells and the increased expression of activation/inhibitory genes such as LAG3, TIGIT, GZMB, GZMK, GZMA, GZMH, NKG7, and EOMES may be important gene expression features associated with treatment resistance. Therefore, the transcriptional features of cells in CAR-T cell products are closely related to clinical efficacy.
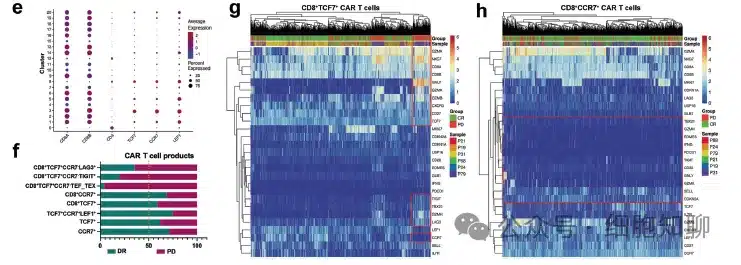
▲Figure 3: Single-cell RNA-seq analysis of infused CAR-T cell products
The composition and function of initially harvested T cells from patients receiving CAR-T cell therapy differ before and after treatment
The authors further validated whether the characteristics of the CAR-T cell products were directly related to the differentiation status and function of the patient’s initial T cells before treatment. The composition and function of initially harvested T cells from 11 resistant patients and 26 long-term responding patients were further analyzed. It was found that the proportion of naive T cells (CD45RA+CCR7+CD62L+) was significantly higher in responding patients than in resistant patients, while the proportions of TEFF cells (CD45RA+CCR7-CD62L-) and TCM cells (CD45RA-CCR7+CD62L+) were significantly increased in resistant patients (Figure 4). These differences were mainly due to the proportion of CD8+ T cells. Simultaneously, the expression levels of GZMB, IFN-γ, and TNF-α in the initially harvested T cells from responding patients were higher than those from resistant patients after stimulating the TCR signal. Furthermore, the bulk RNA-seq analysis of initially harvested T cells showed results similar to those of CAR-T cells, with upregulation of genes involved in immune processes and the activation of IFN-γ and TNF-α-mediated pathways in CD8+ naive T cells from responding patients compared to resistant patients. These data suggest that the differences in antitumor efficacy between CAR-T cell products exist in their initial T cell sources.
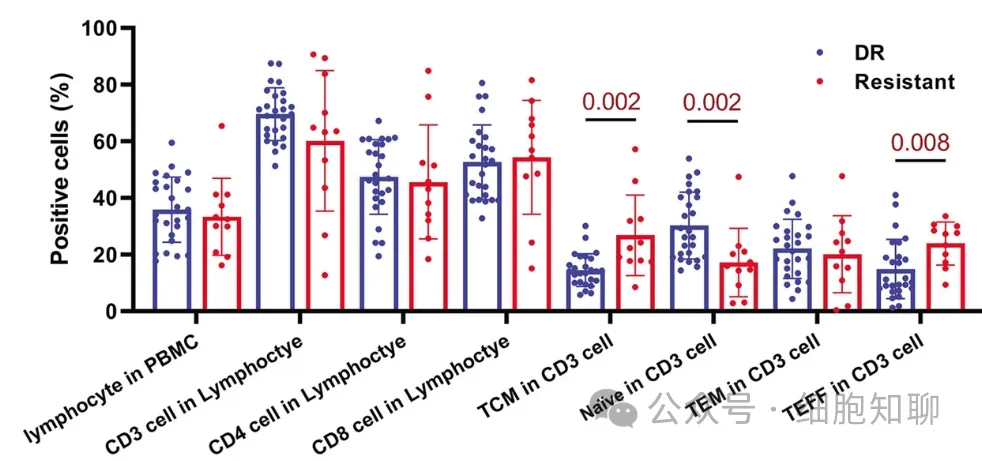
▲Figure 4: Cell population analysis of initially harvested T cells from responding and resistant patients
CD8+ T cell memory features in initially harvested T cells before production are associated with sustained response to CAR-T cell therapy
To better identify and determine the characteristics and relationships between CAR-T cell products and initially harvested T cells, the research team selected initially harvested T cells with the same TCR as the CAR-T cell products from 3 responding and 3 resistant patients, and identified 10 subclusters with different transcriptional features.
The results showed that the relative proportions of T cells in the CD4+ and CD8+ memory/naive clusters were significantly higher in responding patients. The expression of CCR7, LEF1, AIF1, LTB, and LST1 genes was significantly upregulated in cells from responding patients, while the expression of CD69, GZMK, DUSP2, CXCR4, TIGIT, and JUN genes was significantly upregulated in cells from resistant patients. The proportions of non-memory T cell clusters were relatively similar between responding and resistant patients, but the average expression intensity of effector genes and inhibitory genes in initially harvested T cells from resistant patients was higher than that from responding patients.
Subsequently, the researchers analyzed the expression of cells expressing memory-related genes in different patients. The expression intensity of CCR7 in CD4+ and CD8+ naive T cells was higher in responding patients than in resistant patients, while the expression intensity of other memory-related genes showed no difference (Figure 5). The relative proportions of TCF7+ naive T and TCF7+ memory cells in resistant patients were similar to those in responding patients, but the proportions of CCR7+ naive cells and memory cells in initially harvested T cells from responding patients were significantly higher than those from resistant patients. The relative number of CD8+TCF7+CCR7- cells was significantly higher in resistant patients than in responding patients and expressed activation-related genes (including GZMB, GZMA, and IFNG).
This result is consistent with the analysis of the CAR-T product, indicating that the functional memory phenotypic differences in patients’ CAR-T cells exist in the T cells before production, and the CCR7+ T cells in resistant patients show decreased memory function and increased expression of activation and inhibitory genes such as TIGIT and LAG3. Subsequently, CAR-T cells produced from naive T cells were found to be more effective in killing tumor cells than conventional CAR-T cells. These data suggest that the differences in antitumor efficacy between CAR-T cell products may be determined by the quantity and function of naive T cells before production.
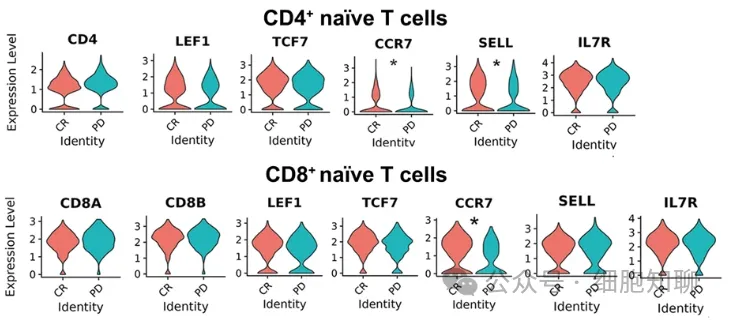
▲Figure 5: Expression of specific genes in CD4+ naive (top) and CD8+ naive (bottom) T cell clusters.
Discussion
In summary, this study found that CAR-T cells from resistant patients had relatively lower antitumor killing, cytokine production, and degranulation abilities in vitro. Subsequently, it was verified whether the antitumor differences between CAR-T cell products from resistant and responding patients were due to the intrinsic properties or cellular composition of T cells. The data showed that there were no differences in the proportions of TEM, TCM, and TEFF cells between responding and resistant patients in the CAR-T cell products, but the proportion of the CD8+TSCM subgroup was significantly higher in responding patients than in other patients.
Therefore, the content and function of CD8+TSCM in CAR-T cells are important factors for patients to achieve long-term remission, and the lack of initial CD8+ naive T cells may lead to resistance to CAR-T cell therapy. Although the efficacy of CAR-T therapy is still difficult to fully elucidate, CD8+ TSCM in CAR-T cells represents a piece of the puzzle in reducing treatment resistance and improving response to CAR-T cell therapy. In the future, by collecting T cells from better sources (e.g., collecting T cells at an early stage of disease diagnosis or cell screening), CAR-T cells with improved function and reduced resistance to CAR-T treatment can be prepared.
Reference:
[1] Wang, Y., Tong, C., Lu, Y. et al. Characteristics of premanufacture CD8+ T cells determine CAR-T efficacy in patients with diffuse large B-cell lymphoma.Sig Transduct Target Ther. 8, 409 (2023).
Content Source:细胞知聊
