On May 2, 2018, FDA Approves Kymriah (Tisagenlecleucel) for Treatment of Adult Patients with Relapsed or Refractory Large B-Cell Lymphoma
On May 2, 2018, FDA Approves Kymriah (Tisagenlecleucel) for Treatment of Adult Patients with Relapsed or Refractory Large B-Cell Lymphoma
On May 2, 2018, the U.S. FDA approved Novartis’ CAR-T cell therapy drug Kymriah (Tisagenlecleucel, CTL019) for a second indication, to treat adult patients with relapsed or refractory large B-cell lymphoma (DLBCL) after two or more lines of systemic therapy, including the most common form of non-Hodgkin lymphoma—diffuse large B-cell lymphoma (DLBCL)—as well as high-grade B-cell lymphoma and DLBCL arising from follicular lymphoma (FL).
It is noteworthy that this is the second indication granted by the FDA for Novartis’ CAR-T cell therapy drug Kymriah. On August 30, 2017, the U.S. FDA had already approved Novartis’ breakthrough CAR-T therapy Kymriah (Tisagenlecleucel, CTL019) for the treatment of patients up to 25 years of age with B-cell precursor acute lymphoblastic leukemia (ALL) that is refractory or in second or later relapse, making it the first-ever approved CAR-T cell product in human history.
CAR-T cell infusion (Image source: Penn)
According to relevant data, DLBCL affects approximately 30% of NHL patients, and there are about 27,000 new cases of DLBCL diagnosed in the United States each year. Of these patients, approximately 6,500 experience relapsed or refractory disease after two or more lines of treatment, and these patients may now be eligible for Kymriah treatment.
As the lead investigator for the clinical trial of this drug, Dr. Stephen J. Schuster from the University of Pennsylvania said: “This is an exciting news, and we see this life-saving therapy can be widely applied to a large unmet medical need, potentially saving many lives.”
Last year at the American Society of Hematology (ASH) annual meeting, Novartis and the University of Pennsylvania (Penn) jointly released key new data on Kymriah (CTL019) from the JULIET study.
In this global pivotal Phase II clinical trial called JULIET, researchers evaluated the efficacy of Kymriah (CTL019) in patients with relapsed/refractory DLBCL, and the clinical results confirmed the durability of this therapy.
Key trial data showed:
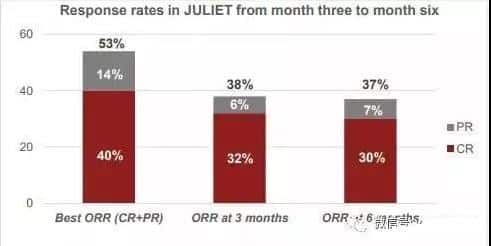
Clinical results (Image source: Novartis)
In 81 patients with a follow-up of 3 months or earlier, the overall response rate (ORR) was 53%, with a complete response rate (CR) of 40% and a partial response rate (PR) of 14%.
At 6 months after Kymriah infusion, the ORR was 37%, and the CR rate was 30%. The median duration of response had not been reached.

Stephen J. Schuster (Image source: ecancer)
Dr. Stephen J. Schuster said, “At enrollment in the trial, these DLBCL patients had received multiple lines of chemotherapy, had a poor prognosis, and had almost no treatment options left. With Kymriah (Tisagenlecleucel) treatment, we have been able to significantly improve their chances of achieving and maintaining a durable response, thus demonstrating the benefits of this therapy in treating this deadly blood cancer.”
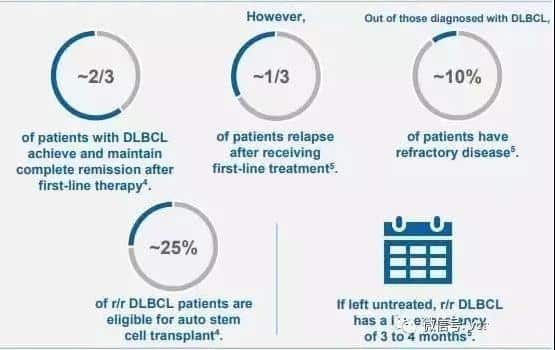
Image source: Novartis
At the 3-month mark, the CR rate was 32%, and the PR rate was 6%, consistent with the 6-month rates (30% CR, 7% PR).
In the JULIET study, the probability of being relapse-free at 6 months after the initial response was 74% (95% CI, 52%-87%), the median duration of response was not reached, and the median overall survival (OS) was also not reached (95% CI: 6.5 months to not estimable), with a median time from infusion to data cutoff of 5.6 months.
Based on this clinical data, the FDA approved Kymriah’s second indication. This is the latest achievement from the alliance between Penn and Novartis, who entered into a global collaboration in 2012 to further research, develop, and commercialize Kymriah and other CAR-T cell therapies for cancer treatment. Researchers at the Perelman School of Medicine at the University of Pennsylvania have led the research, development, and related clinical trials for CAR-T, in collaboration with Novartis. They state that today’s approval of Kymriah’s second indication is a significant step in saving patients’ lives!
Liz Barrett, CEO of Novartis Oncology, said, “For the approval of Kymriah’s two indications, we thank the leadership of Penn in the development and the brave patients who participated in the clinical trials, making Kymriah one of the most exciting anti-cancer technologies to date.”
Content Source:金斯瑞投资者之家
