On May 27, 2022, the FDA Approved Kymriah (Tisagenlecleucel) for the Treatment of Adult Patients with Relapsed or Refractory Follicular Lymphoma (FL) After Two or More Lines of Systemic Therapy
On May 27, 2022, the FDA Approved Kymriah (Tisagenlecleucel) for the Treatment of Adult Patients with Relapsed or Refractory Follicular Lymphoma (FL) After Two or More Lines of Systemic Therapy
On May 27, 2022, Novartis announced on its official website that the U.S. FDA has granted accelerated approval for a new indication of its CAR-T cell therapy Kymriah (tisagenlecleucel) for the treatment of adult patients with relapsed or refractory (r/r) follicular lymphoma (FL) after two or more lines of systemic therapy.
Follicular lymphoma is the second most common subtype of non-Hodgkin lymphoma (NHL), an indolent lymphoma accounting for approximately 22% of all NHL cases, and 8.1%-23.5% of NHL cases in China. According to the guidelines for the diagnosis and treatment of lymphoma, follicular lymphoma is highly prone to relapse, and almost all patients who experience the first relapse will relapse again.
Although current therapies can improve the overall survival of patients, follicular lymphoma is still considered an incurable malignancy because the disease progresses in a pattern of “relapse-remission-re-relapse.” In their lifetime, patients with relapsed follicular lymphoma may receive more than 5 different therapies, up to 12 at most. Therefore, for patients with refractory or relapsed disease, the efficacy of treatment in the later stages is significantly compromised, and they may even face a situation where no effective treatment is available.
Kymriah is a CAR-T cell therapy targeting the CD19 antigen. Previously, it has been approved by the FDA and the European Union for the treatment of relapsed or refractory acute lymphoblastic leukemia and diffuse large B-cell lymphoma.

Clinical Data
This accelerated approval was based on the ELARA trial, a multicenter, single-arm, open-label study evaluating the efficacy of the CAR-T cell therapy Kymriah in patients with relapsed or refractory follicular lymphoma.
As of September 28, 2020, a total of 98 patients were enrolled in the trial, and all patients except one received the CAR-T cell therapy.
The median age of the enrolled patients was 58 years; 31% were female; 78% were White, 10% were Asian, and 1% were Black or African American; the median number of prior therapies was 4, with 24% of patients having received 2 prior lines, 21% having received 3 prior lines, and 54% having received ≥4 prior lines; 87% of patients had stage III-IV disease at the start of the study; 36% had previously undergone autologous stem cell transplantation, 79% were refractory to their most recent regimen, and 66% had progressive disease within 24 months of their first anti-CD20 combination therapy.
The primary endpoint was the complete response (CR) rate as assessed by an Independent Review Committee (IRC). Secondary endpoints included objective response rate (ORR), duration of response (DOR), progression-free survival (PFS), overall survival (OS), and safety.
In the overall population (n=98), the ORR was 86%, and the CR rate was 67%.
In the primary efficacy population (n=90), the ORR was 86%, and the CR rate was 68%; the median DOR was not estimable, with a 9-month DOR rate of 75.2% and a 12-month DOR rate of 70.8%; among patients who achieved CR, 87.7% maintained their response for at least 9 months after initially achieving CR, and 85.2% maintained their response for at least 12 months after initially achieving CR.
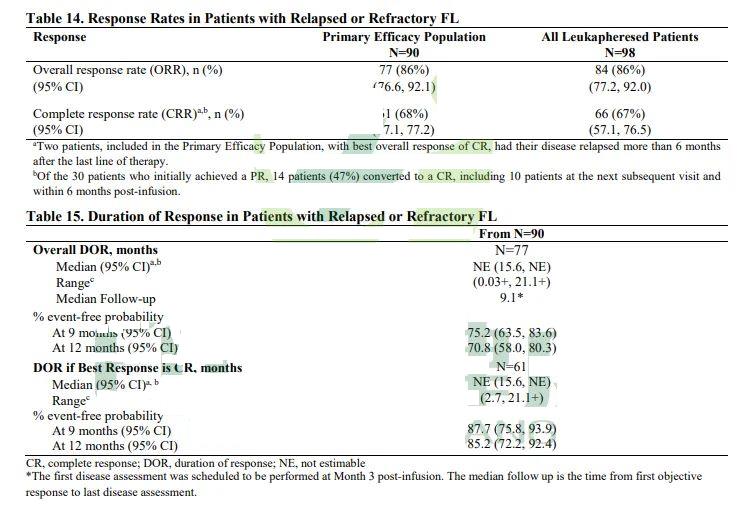
Image note: Clinical data of CAR-T cell therapy Kymriah in the treatment of patients with relapsed or refractory follicular lymphoma
Adverse Reactions
In this trial, the most common adverse reactions of any grade included: cytokine release syndrome (53%), infections of unspecified etiology (38%), fatigue (27%), musculoskeletal pain (25%), headache (25%), diarrhea (24%), fever (19%), cough (19%), hypogammaglobulinemia (18%), viral infectious disorders (18%), nausea (16%), constipation (16%), febrile neutropenia (13%), abdominal pain (10%), arthralgia (10%), rash (10%).
The most common Grade 3 or higher adverse reactions included: febrile neutropenia (13%), infections of unspecified etiology (12%), viral infectious disorders (5%), fatigue (3%), diarrhea (2%), nausea (2%), headache (2%), abdominal pain (1%), fever (1%), hypogammaglobulinemia (1%), musculoskeletal pain (1%).
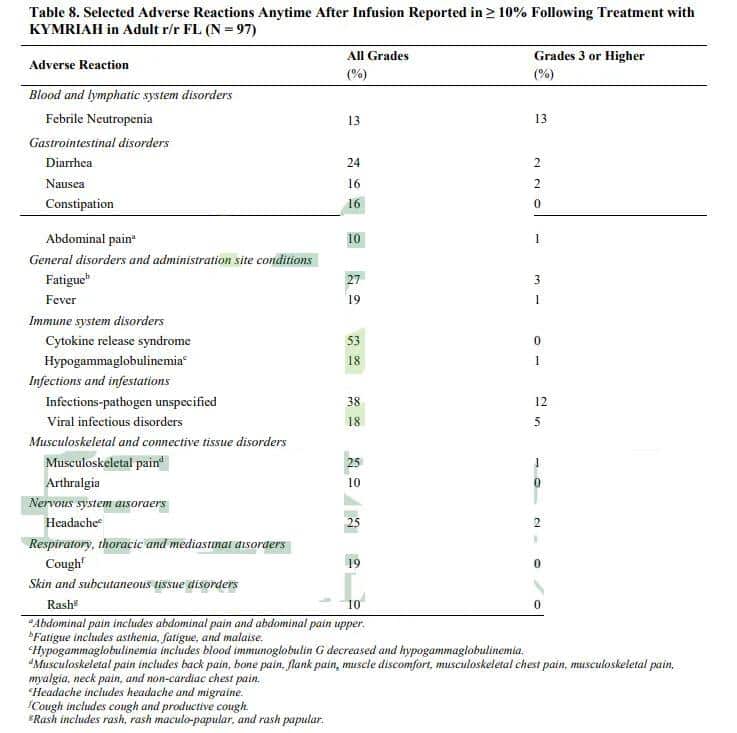
Image note: Adverse reactions of CAR-T cell therapy Kymriah in the treatment of patients with relapsed or refractory follicular lymphoma
The most common Grade 3 or 4 laboratory abnormalities included: neutropenia (63%), leukopenia (40%), thrombocytopenia (21%), anemia (20%), lymphopenia (19%), hypophosphatemia (12%).
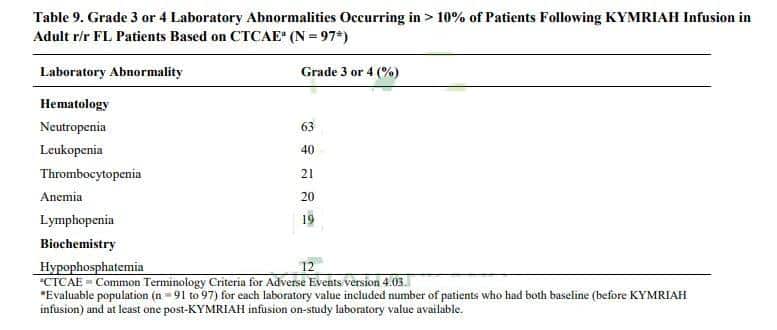
Image note: Laboratory abnormalities of CAR-T cell therapy Kymriah in the treatment of patients with relapsed or refractory follicular lymphoma
Warnings and Precautions
Cytokine release syndrome
Neurological toxicities
Hemophagocytic lymphohistiocytosis/Macrophage activation syndrome
Hypersensitivity reactions
Serious infections
Prolonged cytopenias
Hypogammaglobulinemia
Secondary malignancies
Effects on ability to drive and use machines
Content Source:印塔健康
