October 17, 2022 Yescarta EMA Approved for Second-line Treatment of Diffuse Large B-cell Lymphoma (DLBCL) and Advanced B-cell Lymphoma (HGBL)
October 17, 2022 Yescarta EMA Approved for Second-line Treatment of Diffuse Large B-cell Lymphoma (DLBCL) and Advanced B-cell Lymphoma (HGBL)
On October 17, 2022, exciting news arrived from the European Commission (EC). Based on data from the ZUMA-7 study, the EC officially approved a new indication for Yescarta® for the treatment of adult patients with diffuse large B-cell lymphoma (DLBCL) and high-grade B-cell lymphoma (HGBL) who are refractory to or relapse within 12 months after first-line chemoimmunotherapy. This is the first CAR-T cell therapy product approved in the European Union for second-line treatment of large B-cell lymphoma (LBCL), bringing a new treatment option for lymphoma patients in Europe!
DLBCL is the most common type of non-Hodgkin’s lymphoma (NHL), accounting for approximately 25%-50% of all NHL cases. After first-line treatment, 30%-40% of DLBCL patients may still relapse or become refractory, and once progressed to relapsed or refractory disease, the median survival may be only 6.3 months. HGBL is a heterogeneous disease with morphological and genetic features between DLBCL and Burkitt lymphoma, accounting for 1-2% of all NHL cases. HGBL is highly aggressive, with rapid disease progression, poor prognosis, and poor response to conventional chemotherapy regimens, resulting in a low cure rate. Both DLBCL and HGBL patients have limited benefits from second-line treatment, and there is an urgent need for breakthrough therapies.
Yescarta® (U.S. product generic name: axicabtagene ciloleucel, abbreviated as Axi-Cel) is a gene-modified autologous T-cell immunotherapy targeting CD19. In August 2018, Yescarta® received its first marketing approval in the European Union for the treatment of adult patients with relapsed/refractory (R/R) LBCL after two or more lines of systemic therapy, including DLBCL and primary mediastinal large B-cell lymphoma (PMBCL). In the same year, Yescarta® initiated the ZUMA-7 study to compare the efficacy and safety of Yescarta versus standard treatment for second-line treatment of LBCL. ZUMA-7 is a global, multicenter, randomized phase III study that enrolled 359 patients with LBCL who were refractory to or relapsed within 12 months after first-line chemoimmunotherapy. Patients were randomized 1:1 to receive Yescarta (n=180) or standard treatment (n=179), with the primary endpoint being event-free survival (EFS).
With a median follow-up of 24.9 months, the median EFS was significantly longer in the Yescarta group compared to the standard treatment group (8.3 months vs. 2.0 months, HR: 0.40 [95% CI, 0.31–0.51], P<0.001) (Figure 1). The 24-month EFS rate was also higher in the Yescarta group than in the standard treatment group (41% vs. 16%)[9].
Figure 1: EFS data for Yescarta vs. Standard Treatment

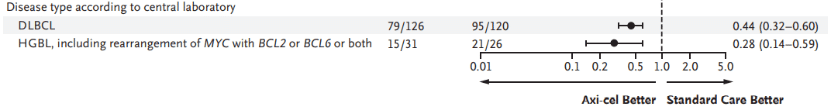
For DLBCL patients, Yescarta reduced the risk of disease progression or death by 56% compared to standard treatment (HR: 0.44 [95% CI, 0.32–0.60]); for HGBL patients (including those with MYC rearrangement with BCL-2 and/or BCL-6 rearrangement), Yescarta reduced the risk of disease progression or death by 72% compared to standard treatment (HR: 0.28 [95% CI, 0.14–0.59]) (Figure 2)[9].
Figure 2: Forest plot of key subgroups for Yescarta vs. Standard Treatment
The safety results of Yescarta in the ZUMA-7 study were consistent with previous related studies. The incidence of grade ≥3 adverse events was 91% in the Yescarta group and 83% in the standard treatment group. Among patients receiving Yescarta treatment, the incidence of grade ≥3 cytokine release syndrome (CRS) and neurological toxicity events were 6% and 21%, respectively (Table 1), with no deaths due to CRS or neurological toxicity[9].
Table 1: Incidence of adverse events for Yescarta vs. Standard Treatment
| Yescarta(N=170) | Standard Treatment (N=168) | |||
| Event N(%) | ANY LEVEL | ≥ Level 3 | ANY LEVEL | ≥ Level 3 |
| Any adverse event | 170(100) | 155(91) | 168(100) | 140(83) |
| CRS | 157(92) | 11(6) | – | – |
| Neurotoxicity | 102(60) | 36(21) | 33(20) | 1(1) |
Based on the significant survival benefit observed in the ZUMA-7 study, in April 2022, Yescarta® received FDA approval for the new indication of second-line treatment of LBCL (for the treatment of adult patients with LBCL who are refractory to or relapse within 12 months after first-line chemoimmunotherapy), followed by the recent approval from the European Union.
