Am J Hematol | Long-term Remission Rates in Real-World “CAR-T Cell CNS Lymphoma” Patients
Am J Hematol | Long-term Remission Rates in Real-World “CAR-T Cell CNS Lymphoma” Patients
Primary central nervous system lymphoma (PCNSL) is a central nervous system-confined non-Hodgkin lymphoma (NHL), with the pathological diagnosis being mainly diffuse large B-cell lymphoma. It is confined to the brain, spinal cord, cranial nerves, leptomeninges, and vitreoretina, a relatively rare tumor accounting for approximately 1% of NHLs. The treatment primarily involves chemotherapy and radiation therapy, but the overall efficacy and prognosis are poor, especially for relapsed/refractory PCNSL (r/r-PCNSL). With the advent and continuous progress of CAR-T cell therapy, its application in r/r-PCNSL has become a research hotspot. Recently, Professor Sylvain Choquet et al. from France published a study in the American Journal of Hematology, analyzing the efficacy and safety of anti-CD19 CAR-T cells in r/r-PCNSL based on the French Eye-Brain Lymphoma Network (LOC) database.
Background
Primary central nervous system lymphoma (PCNSL) is an extremely rare type of diffuse large B-cell lymphoma (DLBCL) with a high risk of relapse, leading to a poor prognosis with a median overall survival (OS) of only 6.8 months. Research data show that after salvage treatment, high-dose chemotherapy followed by autologous stem cell transplantation (ASCT) can improve the survival of patients with relapsed/refractory primary central nervous system lymphoma (r/r-PCNSL). However, in a real-world study in France involving 1002 PCNSL patients between 2011 and 2016, only 17% of relapsed PCNSL patients benefited from ASCT treatment, with a 5-year OS of 52% in these patients. The low proportion of patients benefiting from ASCT is mainly due to advanced age, comorbidities, or ineffective salvage treatment. Additionally, while some other treatment regimens can achieve a high objective response rate (ORR) exceeding 50%, the response duration is usually short.
In recent years, the emergence of anti-CD19 CAR-T cells has brought significant progress in the treatment of DLBCL, allowing 40-50% of relapsed PCNSL patients after second-line therapy or later to achieve long-term remission. In the second-line treatment of early relapsed or refractory DLBCL patients, both axicabtagene ciloleucel (axi-cel) and lisocabtagene maraleucel (liso-cel) have shown superior efficacy compared to salvage treatment followed by ASCT. These results led to the approval of axi-cel and tisa-cel for the treatment of r/r-DLBCL patients who have received at least two prior lines of therapy, and commercial production. Recently, axi-cel and liso-cel have also been approved for second-line treatment of early relapsed or refractory DLBCL.
Currently, there are few clinical studies on CAR-T cell therapy in PCNSL patients. A recent meta-analysis involving 30 PCNSL patients showed that the complete response rate (CR) with CAR-T cell therapy was 56%, the 6-month progression-free survival (PFS) rate was 37%, and neurotoxicity was relatively manageable, with an immune effector cell-associated neurotoxicity syndrome (ICANS) incidence of 53%, of which 18% were grade 3-4. This study aims to elucidate the value of CAR-T cell therapy in the treatment of PCNSL through a larger cohort and longer follow-up period.
Patients and Study Methods
The PCNSL patients in this study were from the LOC database, with the following inclusion criteria:
1) PCNSL patients aged 18-80 years;
2) Initial diagnosis between 2011-2021;
3) First-line treatment based on a high-dose methotrexate (HD-MTX) regimen;
4) Presence of central nervous system relapse but no systemic involvement;
5) Ineligible for ASCT or relapse after ASCT;
6) Received any treatment other than ASCT or CAR-T for the current relapse.
Exclusion Criteria
Patients receiving palliative treatment at relapse were excluded. Treatment response was evaluated using the IPCG criteria, while cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) were defined according to the ASTCT 2019 guidelines.
Results
The study included 27 r/r-PCNSL patients, of whom 25 eventually received CAR-T cell therapy, with a median age of 68 years. On average, they had received 3 prior lines of treatment (14 had undergone ASCT, and 2 had received whole-brain radiation therapy). In 22 (81%) cases, there was brain parenchymal involvement, and in 8 (30%) cases, there was cerebrospinal fluid involvement. Only 1 patient did not receive bridging therapy.
With a median follow-up of 20.8 months (19.4 months after CAR-T cell infusion), the results showed:
1) The median progression-free survival (mPFS) reached 8.4 months, with 6-month and 12-month PFS rates of 63% and 43%, respectively. Taking CAR-T cell infusion as the starting point, the 12-month PFS was 46% (see Figure 1).
2) Eleven patients relapsed after CAR-T cell therapy, of whom 7 received at least one additional treatment, including 2 who received whole-brain radiation therapy (WBRT). After 4.6 months, all but one died due to lymphoma progression.
3) Among the 16 patients who achieved complete response (CR), only 2 subsequently relapsed.

Figure 1. progression free survival (PFS)
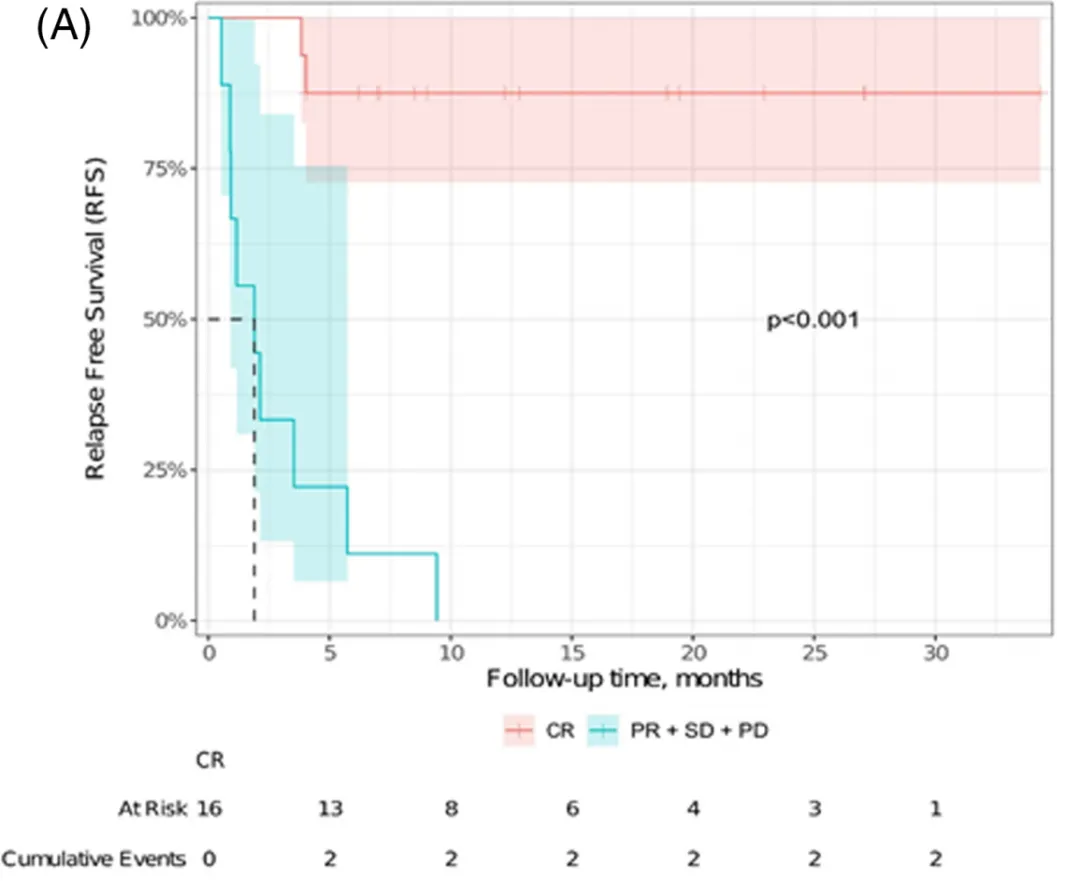
Figure 2. recurrence free survival (RFs)
4) Comparing patients who achieved CR and those who did not after CAR-T cell therapy, the 12-month relapse-free survival (RFS) rates were 87% vs. 0% (P<0.001), as shown in Figure 2.
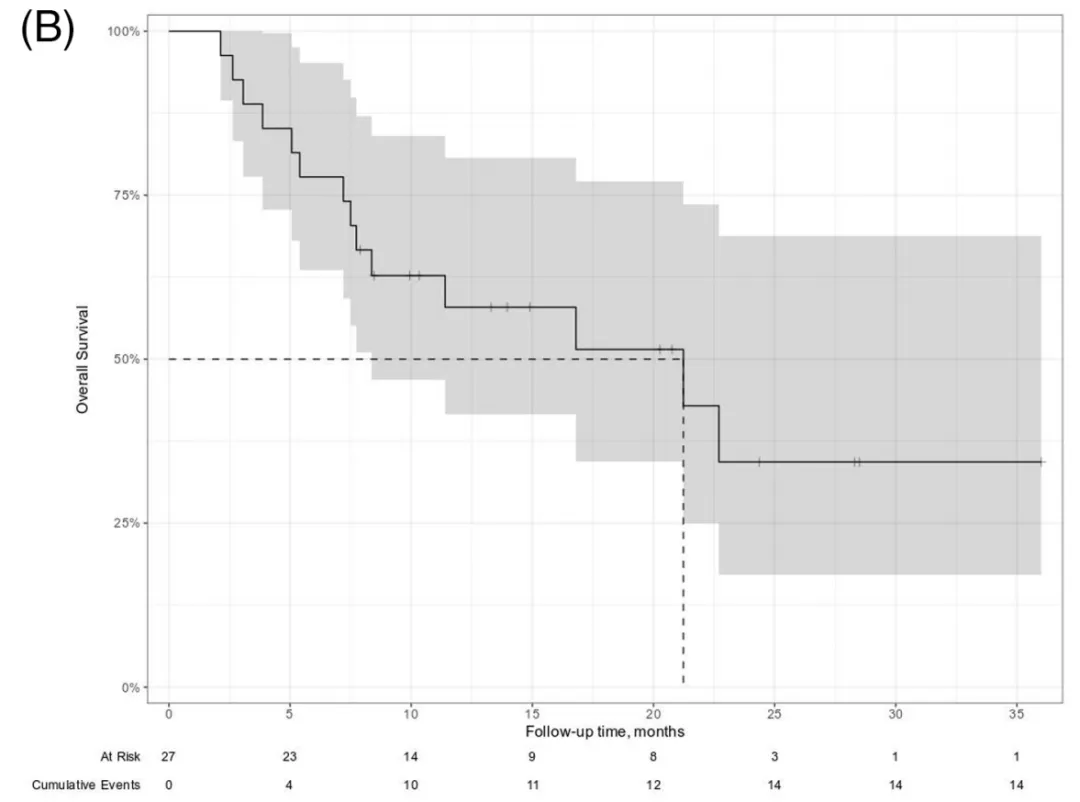
Figure 3. overall survival (OS)
5) The median OS was 21.2 months, with 6-month, 12-month, and 18-month OS rates of 77%, 58%, and 51%, respectively (Figure 3). Taking CAR-T cell infusion as the starting point, the 12-month OS was 55%. Twelve patients died after CAR-T cell therapy, 10 due to lymphoma progression, 1 due to COVID-19 infection, and 1 due to neurotoxicity.
6) Regarding safety, 23 (92%) patients experienced cytokine release syndrome (CRS) after CAR-T cell therapy, with 2 cases being grade 3-4. Seventeen (68%) patients experienced ICANS after CAR-T cell therapy, including 8 with axi-cel and 9 with tisa-cel, with 5 (20%) cases being grade ≥3. The average time to CRS and ICANS onset after CAR-T cell infusion was 1 day and 5 days, respectively, with an average resolution time of 4 days and 6 days, respectively. Additionally, to manage CAR-T-related toxicities, 18 (72%) and 14 (56%) patients received tocilizumab and steroids, respectively.
Conclusion
This series study is currently the largest cohort study on CAR-T cell therapy for PCNSL, and its results confirm that anti-CD19 CAR-T cells are effective in heavily pretreated r/r-PCNSL patients, achieving high response rates, with a complete response (CR) rate of up to 86%. In the third-line or later setting, 46% of r/r-PCNSL patients achieved long-term remission, which is better than previously observed results. Although the incidence and severity of neurotoxicity may be slightly higher than in DLBCL patients receiving CAR-T cell therapy, it is generally acceptable in most cases and should not preclude the use of CAR-T cells in PCNSL.
