Can Patients Recover Normal Life after CAR-T Cell Lymphoma Treatment: Examining the Research Findings
Can Patients Recover Normal Life after CAR-T Cell Lymphoma Treatment: Examining the Research Findings
Chimeric antigen receptor T cells (CAR-T cells) can induce long-term remissions in many patients with relapsed/refractory lymphomas. However, little is known about the lives of patients after CAR-T cell therapy.
Recently, the Department of Hematology at the University Hospital of Rennes in France published a journal review titled “Remission After CAR-T Cell Therapy: Can Lymphoma Patients Return to a Normal Life?” in the medical journal HemaSphere.

This prospective review assessed the multidimensional recovery (physical, psychological, social, and occupational) of lymphoma patients in remission, before leukapheresis, before CAR-T cell infusion, and at 3, 6, and 12 months after infusion. Multiple clinical studies showed that at 12 months after CAR-T cell therapy, 81.8% of the 22 evaluable patients were satisfied with their daily life. Nearly half of the patients had regained their pre-diagnosis levels of physical activity, occupation, sexual function, and overall well-being. We found improvements in patients’ health-related quality of life (HRQoL) after CAR-T cell therapy, including anxiety, depression, sexual satisfaction, and overall well-being.
Introduction
Chimeric antigen receptor T cell (CAR-T cell) therapy has revolutionized the treatment of relapsed and refractory (R/R) lymphomas such as large B-cell lymphoma (LBCL), mantle cell lymphoma (MCL), and follicular lymphoma (FL). The long-term remissions induced by CAR-T cell therapy raise the question of whether patients can potentially regain a “normal life” after this treatment.
In clinical trials of CAR-T cell therapy, health-related quality of life (HRQoL) is typically assessed as a secondary endpoint. In the JULIET study, LBCL patients who received tisagenlecleucel (tisa-cel) after at least two lines of systemic therapy showed clinically meaningful improvements in most HRQoL aspects. In the TRANSCEND trial, lisocabtagene maraleucel (liso-cel) infusion improved the overall HRQoL of R/R LBCL patients, with clinically meaningful changes compared to baseline at month 2.
However, patients participating in clinical trials may not represent those treated in the real world. Only two real-world studies have evaluated overall HRQoL months after CAR-T cell infusion. They reported an initial decline in HRQoL within the first 2 weeks, followed by a recovery to baseline within 3 months. However, the first study found no clinically meaningful improvement over time. The second study showed improvements at 3 and 6 months, but the subjects were non-responders, who do not represent patients in remission. Both studies involved heterogeneous populations of patients with multiple myeloma and acute leukemia. Finally, their limited 6-month follow-up could not estimate the recovery to daily life.
Here, we conducted a prospective study in the real-world setting to assess the multidimensional recovery (physical, psychological, social, and occupational) of lymphoma patients in remission during the first year after CAR-T cell therapy.
Methods
Study Design and Data Collection: CARAMA is a prospective, dual-center study conducted at the University Hospitals of Rennes and Toulouse, France. Eligible patients had to be ≥18 years old, able to provide informed consent, and have lymphoma meeting criteria for CAR-T cell therapy. Patients were enrolled before leukapheresis. Bridging chemotherapy was determined by the treating physician.
Disease characteristics were collected from electronic medical records, and sociodemographic data were self-reported by patients. All analyzable patients were requested to complete each questionnaire, collected by dedicated hematology nurses. Clinical data and self-reported questionnaires were collected before leukapheresis, before CAR-T cell infusion, and at 3, 6, and 12 months after infusion. Patient follow-up continued until month 12, study completion, disease progression/relapse, or death.
Long-term follow-up data were obtained through telephone interviews for patients who remained in remission beyond 12 months and were still being monitored by the investigators up to 24 months. These structured interviews covered occupational activity, social life, physical activity, sexual life, and overall well-being.
Assessment Tools: Validated tools were used to measure lymphoma-related and overall HRQoL (Functional Assessment of Cancer Therapy-Lymphoma [FACT-Lym] and EQ-5D-5L), cognitive symptoms (FACT-Cog), fatigue (FACIT-Fatigue subscale), psychological status (Hospital Anxiety and Depression Scale, Post-traumatic Checklist Scale), and relational and sexual functions (Relationship and Sexuality Scale).
Results
Baseline Patient Sample Characteristics: From March 2020 to August 2022, 59 patients were enrolled in the study. The median follow-up time after CAR-T cell infusion was 11 months. Questionnaire completion details are shown in Figure 1.
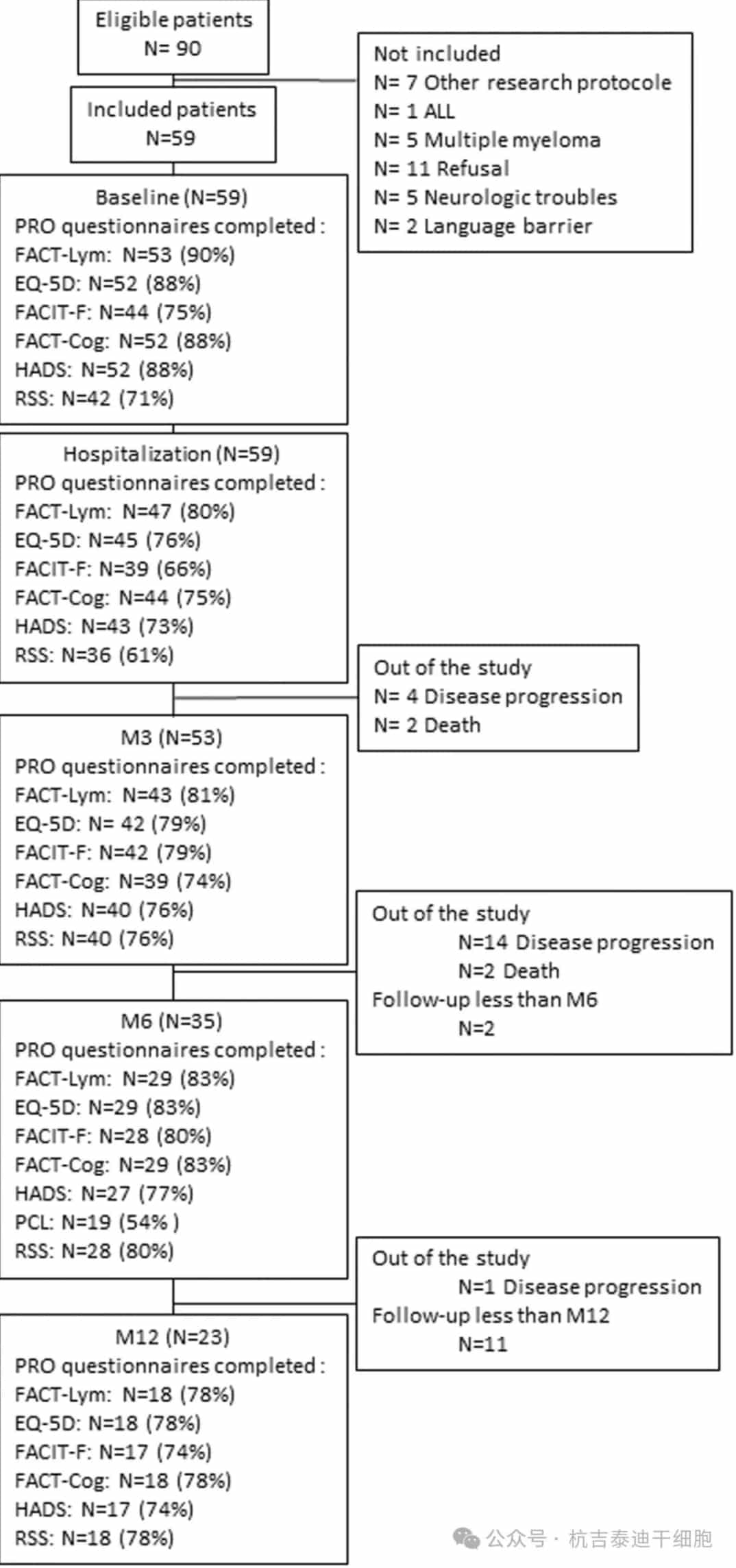
Figure 1: Patient flow and completion rates. Patients were followed until death, disease progression, or study completion. A questionnaire was considered completed if at least one score could be computed. ED-5Q-DL, EuroQol 5 dimension 5 level; EPCL, Post-traumatic Checklist Scale; FACIT-F, Functional Assessment of Chronic Illness Therapy-Fatigue subscale; FACT-Cog, Functional Assessment of Cancer Therapy-Cognitive; FACT-Lym, Functional Assessment of Cancer Therapy-Lymphoma; HADS, Hospital Anxiety and Depression Scale; PRO, patient-reported outcome; RSS, Relationship and Sexuality Scale questionnaire.
During the study, 19 patients (32.2%) progressed, and 4 patients (6.8%) died. At study completion, 2 (3.4%) and 11 (18.4%) patients had their last follow-up at 3 and 6 months, respectively, with the remaining 23 patients (39%) evaluable at month 12.
Patient Characteristics: The median age was 63 years (range: 19–78 years), with 28 men (47.5%) and 31 women (52.5%). Most patients had received two prior lines of therapy (N=39, 66%). Primary diagnoses were LBCL (N=44, 73.6%), FL (N=8, 13.6%), and MCL (N=5, 8.5%).
Quality of Life
We found an improvement in lymphoma-related HRQoL over time. The FACT-LymTS showed clinically meaningful HRQoL improvements at 3, 6, and 12 months after CAR-T cell infusion, with mean changes from baseline of 10.94, 12.16, and 11.72, respectively. The FACT-G showed a significant improvement at 6 months compared to baseline, with raw scores reaching the normal population level at 3 months.
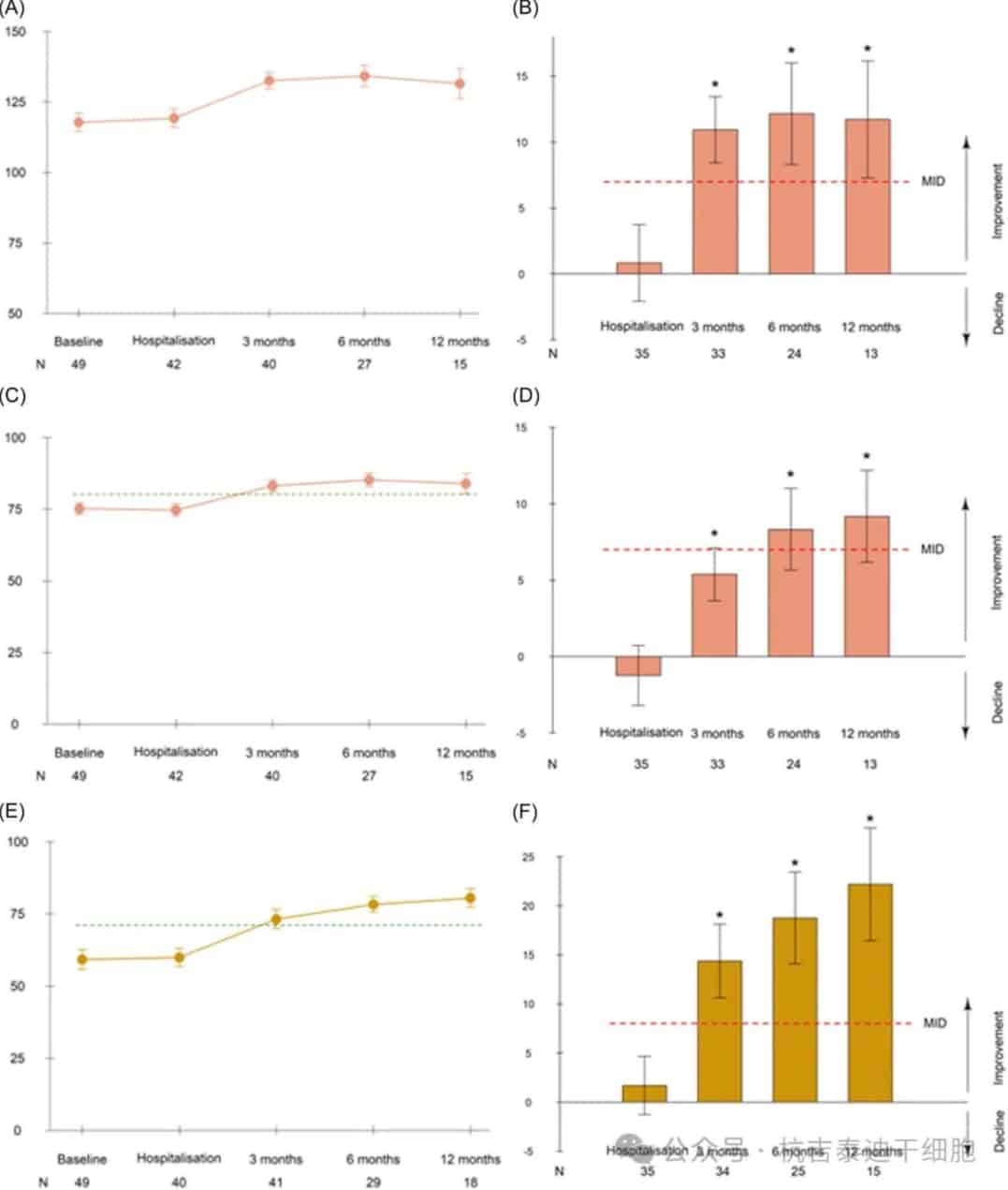
Figure 2: Mean lymphoma-related and overall health-related quality of life scores over time and mean changes from baseline. Mean Functional Assessment of Cancer Therapy (FACT)-Lymphoma total scores (A, B), FACT-General (C, D), and EQ-5D visual analog scale (E, F) scores over time and mean changes from baseline (with standard errors). Before leukapheresis, before CAR-T infusion, and at 3, 6, and 12 months after infusion.
The EQ-5D-5LVAS showed clinically meaningful and statistically significant improvements from 3 to 12 months after infusion (Figure 2). Mean EQ-5DVAS scores increased over time, reaching the reference level for the general population by month 3. The proportion of patients reporting severe problems or inability decreased in almost all domains. At enrollment, 48 patients (69.1%) experienced pain. At 6 and 12 months after infusion, approximately half of the patients still had mild to severe pain (50% and 55.6%, respectively). Notably, patients who experienced pain at 6 and 12 months generally had pain at baseline (84.6% and 88.9%, respectively).
Cognition and Fatigue
The FACT-Cog TS did not show any clinically relevant changes over time (Figure 3). No changes were observed according to the occurrence of ICANS. However, a significant decline in FACT-Cog TS compared to baseline was observed in 6 (19.4%), 3 (13.6%), and 5 (33.3%) patients at 3, 6, and 12 months, respectively. The mean raw scores were similar to normal values in the general population but showed a slight decrease at 12 months.
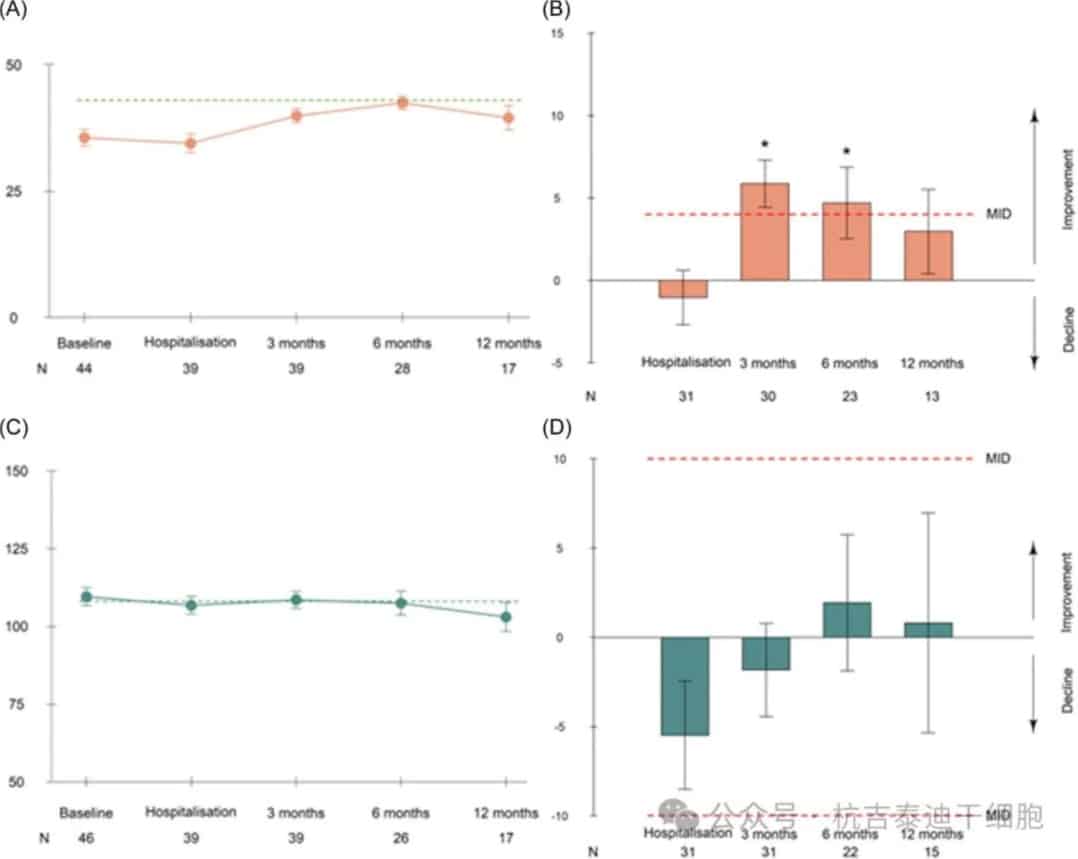
Figure 3: Mean fatigue and cognitive scores over time and mean change relative to baseline. Mean scores of the Functional Assessment of Cancer Therapy (FACT)-Fatigue subscale (A, B) and FACT-Cognitive Total Score (C, D) over time and mean change (with standard error) relative to baseline. Before leukapheresis, before chimeric antigen receptor T-cell infusion, and 3, 6, and 12 months after infusion.
The FACIT-F scale showed clinically relevant improvements in cancer-related fatigue at 3 and 6 months, but no improvement at 12 months. The mean raw scores increased in the first 6 months after infusion, then tended to decrease and were lower than the reference levels in the general population at each time point. At 6 and 12 months, 21.4% and 35.3% of patients, respectively, reported moderate to severe fatigue.
Psychological State
At baseline, 43.1% of patients exhibited moderate or severe anxiety disorders (i.e., a score ≥8), as measured by the HADS questionnaire (Figure 4).
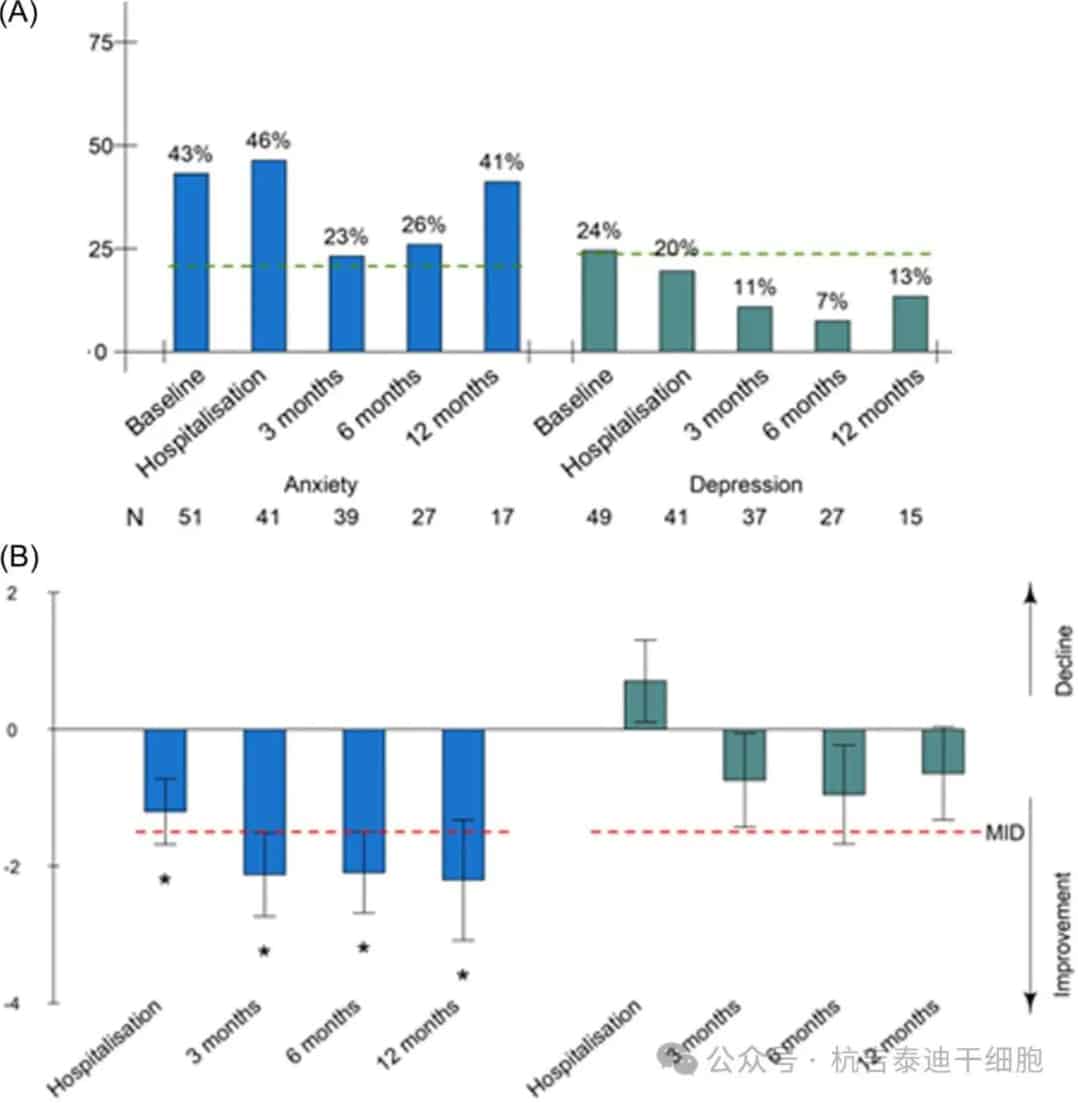
Figure 4: Proportion of patients with anxiety or depressive disorders according to the Hospital Anxiety and Depression Scale (HADS) subscales and mean change from baseline. (A) Proportion of patients with anxiety or depressive disorders according to the HADS subscales and (B) mean change (with standard error) in HADS subscales from baseline. Before leukapheresis, before chimeric antigen receptor T-cell infusion, and 3, 6, and 12 months after infusion.
This proportion remained higher than in the general population (lowest at 23% at 3 months). Anxiety scores showed clinically relevant improvements in anxiety symptoms between 6 and 12 months, reaching statistical significance at 3 months. The 7 patients who maintained moderate or severe anxiety at 6 and 12 months tended to be younger, with a median age of 63 years, and 85.7% had baseline anxiety disorders. The proportion of patients with moderate or severe depressive symptoms (i.e., a score ≥8) decreased from 24.4% at baseline to 13% at 12 months, falling below the general population at 3 months. However, changes in depressive scores over time were not clinically relevant.
At 6 months, 26% of patients had a PCLS score ≥34 (indicating a need for medical care), and 21.3% of patients met the diagnostic criteria for PTSD (i.e., a PCLS score >44).
At 6 months, patients with baseline anxiety or PTSD symptoms had a lower improvement in FACT-LymTS from baseline (Figure 5). Age, sex, and prior lines of therapy were not associated with differential HRQoL improvement from baseline.
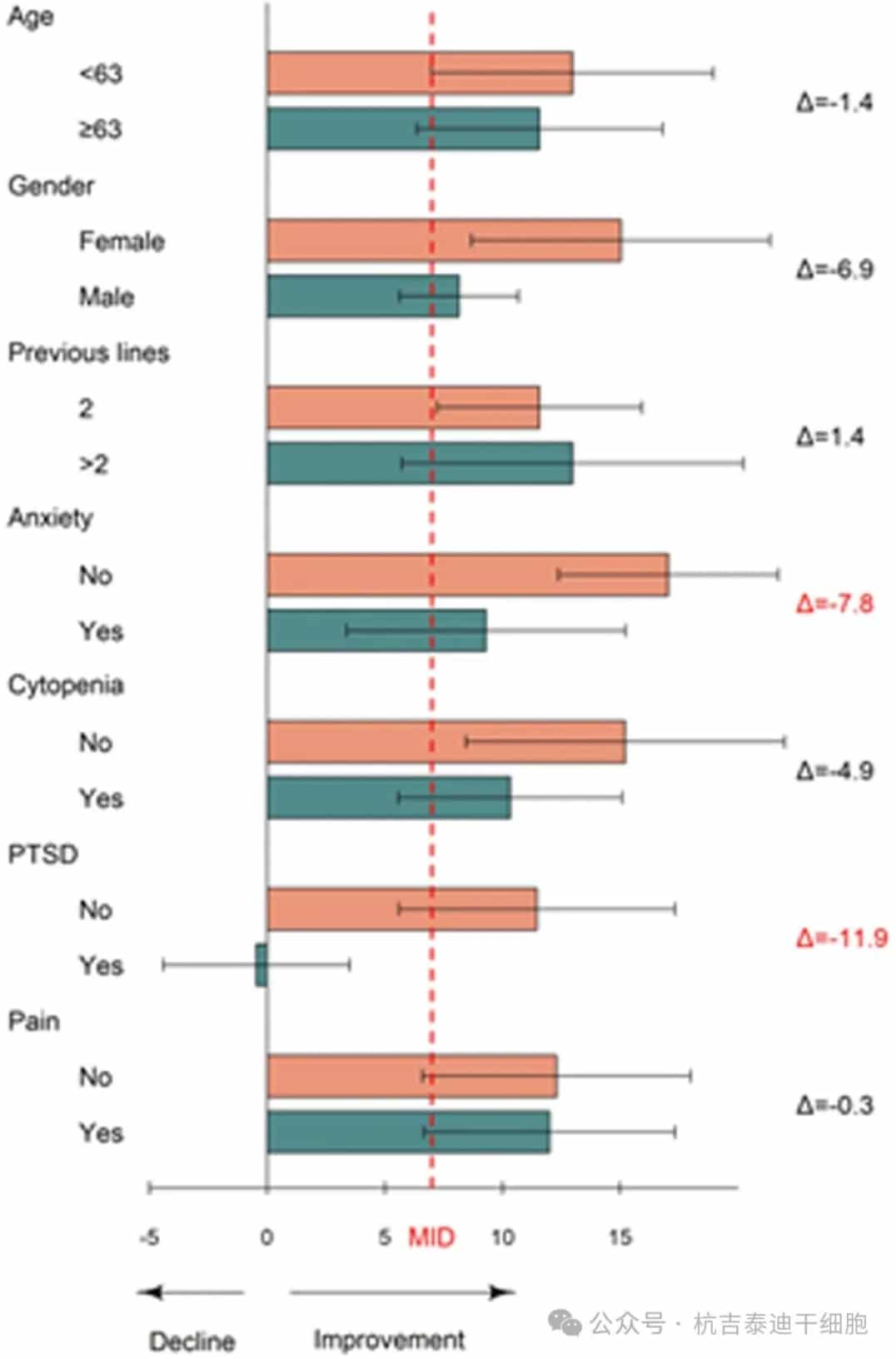
Figure 5: Subgroup analysis of mean change in Functional Assessment of Cancer Therapy-Lymphoma Total Score (FACT-LymTS) at 6 months. Mean change (with SD) in FACT-LymTS at 6 months relative to baseline according to baseline anxiety symptoms, sex, age, cytopenias at 3 months, pain at 6 months, and post-traumatic stress disorder at 6 months.
Sexual Health
The RSS showed that sexual outcomes, including sexual desire, ability to reach orgasm, frequency of hugging and kissing, satisfaction with sexual frequency, and sexual frequency itself, improved at 6 months. At 12 months, 68.2% of evaluable patients (N=22) reported being satisfied with their sex life. Furthermore, 77.3% of patients reported that CAR-T cell therapy had a stable or positive impact on their sex life, and 59% reported it as similar or better than before their lymphoma diagnosis. Only 6.8% of patients discussed their sex life with a healthcare provider.
Occupational, Social, and Physical Recovery
After a median follow-up of 19 months, 22 patients (37.3%) remained in remission for at least 12 months.
Fewer than half of the patients were occupationally active at the time of lymphoma diagnosis. For patients in remission at 12 months (Figure 6), among the 10 patients of working age (i.e., <62 years old at CAR-T cell infusion), half had returned to work (N=5), with 3 being full-time employees. Overall, 20.8% of patients aged <62 years at CAR-T cell infusion resumed occupational activity after CAR-T cell therapy. All patients were satisfied with their current occupational activity.
The main reason for not returning to work was fatigue: approximately 60% of patients cited it as the reason for continuing medical leave, and one patient still experienced severe pain related to the initial disease.
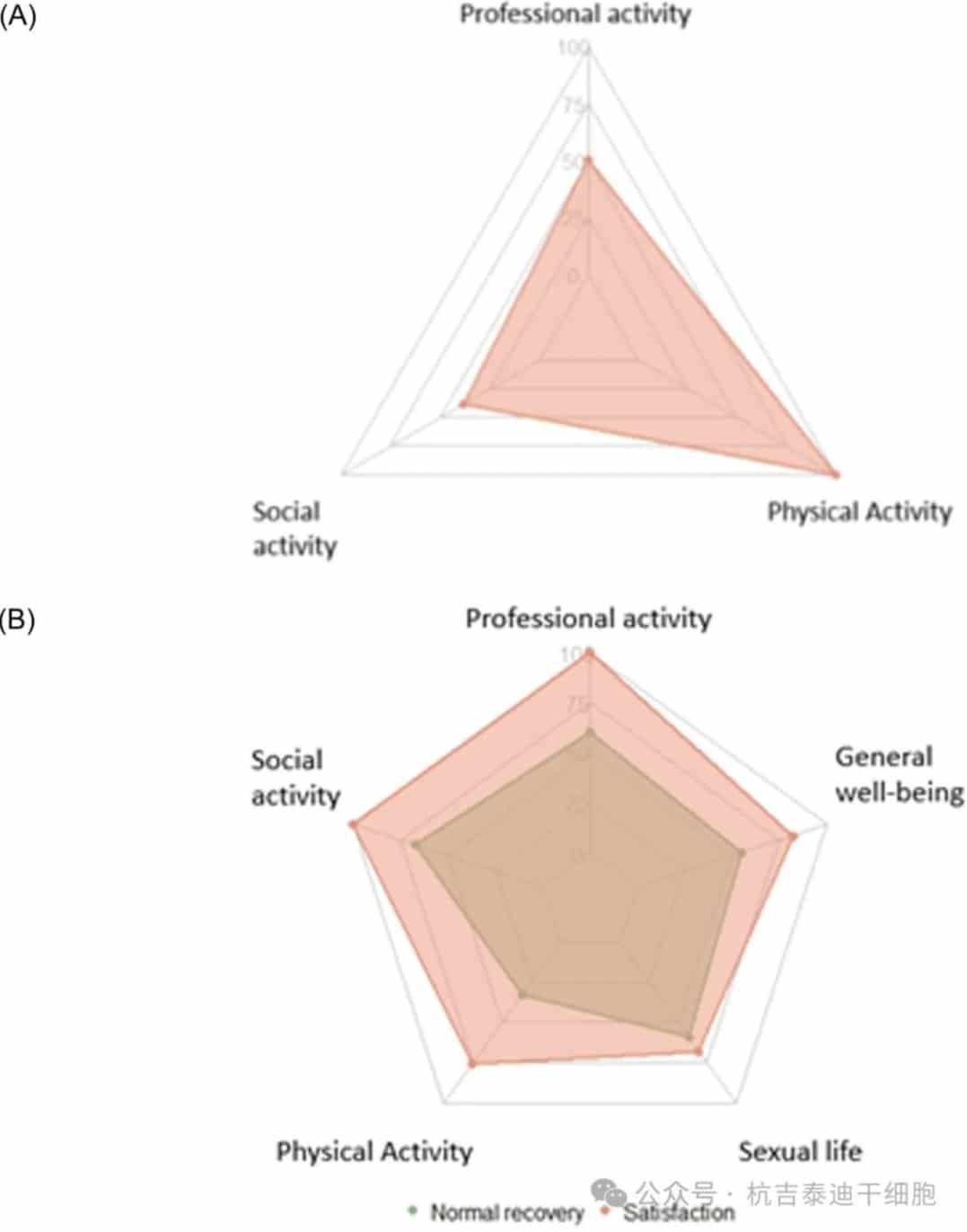
Figure 6: Recovery of daily activities in patients in remission at 12 months (N=22). (A) Proportion of patients who recovered physical activity (N=21), social activity (N=8), and occupational activity (N=10). (B) Proportion of patients who reported normal recovery (stable or better than before lymphoma diagnosis) of physical activity (N=21), social activity (N=3), occupational activity (N=5), sex life (N=22), and overall well-being (N=22). Proportion of patients quite or very satisfied with their current physical, social, and occupational activities, sex life, and overall well-being at follow-up.
Among the 22 patients under long-term monitoring, all but one patient had engaged in physical exercise before their initial diagnosis. Of those who had previously engaged in physical exercise, all patients resumed physical exercise after CAR-T cell therapy, albeit at a lower intensity: 34.6% of patients spent more than 6 hours per week exercising before lymphoma diagnosis, whereas all patients spent less than 6 hours per week exercising after CAR-T cell therapy.
Overall, 66% of patients felt their physical condition was not as good as before their initial diagnosis, but most patients (76%) reported their physical condition as stable or improved compared to before CAR-T cell therapy. Among those who had engaged in social activities (i.e., leisure or community activities) before their lymphoma diagnosis (35.3%), 37.5% resumed such activities after CAR-T cell therapy.
Most patients (77.3%) reported an improvement in their overall health status compared to before CAR-T cell therapy. Overall, 81.8% of patients were satisfied with their overall health status, and 54.5% felt their daily lifestyle had returned to a level close to before their lymphoma diagnosis.
Discussion
Here, we aimed to conduct a comprehensive assessment of HRQoL recovery in lymphoma patients achieving remission after CAR-T cell therapy. To our knowledge, this is the first study to assess patient recovery in a multidimensional manner (physical, psychological, social, sexual, and occupational) and with such long-term follow-up. Our target population included unselected patients from various socioeconomic and demographic backgrounds.
We prospectively assessed PROs using well-validated and designated questionnaires, which were then compared to population norms.
Comparisons revealed that at 12 months post-remission, we also investigated physical, occupational, sexual, and general life status. At 3, 6, and 12 months, 53, 35, and 23 patients were evaluable, respectively. Clinically meaningful improvements in lymphoma-related HRQoL were observed at 3, 6, and 12 months, with mean changes from baseline of 10.9, 12.2, and 11.72, respectively. Clinically meaningful improvements were observed in overall HRQoL, fatigue, and anxiety, but 20%–40% of patients experienced persistent fatigue, psychological distress, and cognitive issues over time. At 12 months post-CAR-T cell therapy, 81.8% of the 22 evaluable patients were satisfied with their daily life.
Our study provides new insights into the recovery and well-being of patients after CAR-T cell therapy.
First, to our knowledge, this is the first study to prospectively assess psychological distress, PCI, and fatigue using specific questionnaires (such as FACT-Cog and FACIT-F), providing a more precise evaluation compared to the non-specific EORTC questionnaires used in other studies.
Second, the assessment was conducted over an extended period (up to 12 months), which has not been done in previous real-world studies.
Third, by comparing the HRQoL questionnaires to norms in the general population, we were able to evaluate whether various parameters recovered to “normal” levels. Fourth, we used a specific questionnaire (RSS) for the first time to assess sexual function after CAR-T cell therapy.
Finally, our study is the first to evaluate physical, social, and occupational recovery one year after CAR-T cell therapy. In fact, assessing whether patients can resume their professional careers after CAR-T cell therapy is crucial, as 44% of them were under 60 years of age.
In summary, we found that CAR-T cell therapy improved the overall quality of life for lymphoma patients achieving remission, enabling approximately half of the patients to resume their daily lives. However, some patients experienced long-term sequelae, and not all patients were able to recover a “normal” or “satisfactory” life. Some symptoms may persist, such as fatigue, anxiety, depression, pain, and impaired sexual health. Many of these symptoms may go undiagnosed without dedicated investigation.
Physicians should be aware of these potential symptoms to manage them effectively and improve patients’ health status after CAR-T cell therapy. Further research is needed to identify factors associated with patient recovery after CAR-T cell therapy. This is crucial for improving patient management to facilitate and accelerate comprehensive recovery, such as providing dedicated rehabilitation care or psychological support during the CAR-T cell procedure.
Content Source:杭吉泰迪干细胞
