[2024 EHA] Excellent Efficacy of CAR-T Cell Yescarta in Patients with Relapsed or Refractory Primary Mediastinal Large B-Cell Lymphoma: A Study from the French Registry
[2024 EHA] Excellent Efficacy of CAR-T Cell Yescarta in Patients with Relapsed or Refractory Primary Mediastinal Large B-Cell Lymphoma: A Study from the French Registry
The 2024 European Hematology Association (EHA) Annual Meeting was held in a hybrid format (online and in-person) in Madrid, Spain, from June 13 to 16, Central European Summer Time. As the largest international conference in the field of hematology in Europe, it draws over 10,000 professionals from more than 100 countries worldwide each year to share and discuss the latest diagnostic and therapeutic technologies as well as clinical practice results in the field of hematology[1].
Primary mediastinal B-cell lymphoma (PMBCL) is a rare subtype of large B-cell lymphoma (LBCL), accounting for approximately 4%. It has a favorable outcome with first-line immunochemotherapy[2,3], with 80%-90% of patients achieving long-term survival with the first-line CD20 antibody plus CHOP regimen (R-CHOP). However, some patients fail to achieve complete remission with standard treatment approaches. Relapse mostly occurs within 2 years after treatment completion[4]. Salvage chemoradiotherapy followed by high-dose therapy and autologous stem cell transplantation (ASCT) is effective for most relapsed or refractory primary mediastinal large B-cell lymphoma (R/R PMBL) patients, but alternative strategies are needed for a significant proportion of high-risk patients who are not candidates for cure with this approach[5].
The editor has selected an “Oral Presentation” (S240)[2] to provide insights into the efficacy and toxicity of anti-CD19 autologous chimeric antigen receptor T (CAR-T) cell therapy in patients with relapsed or refractory primary mediastinal B-cell lymphoma (R/R PMBL), and to investigate factors associated with treatment outcomes.
Abstract Number: S240[2,3]
English Title: Excellent outcome of patients with relapsed or refractory primary mediastinal b cell lymphoma treated with axicabtagene ciloleucel: a study from the french DESCAR-T national registry.
Background
There is limited literature studying the outcomes of R/R PMBL patients. Moreover, while chimeric antigen receptor autologous T-cell (CAR-T) therapy has shown impressive complete response (CR) rates and progression-free survival (PFS) in relapsed or refractory large B-cell lymphoma (R/R LBCL), reports on the efficacy of this treatment in PMBL remain scarce. This study aimed to report the efficacy and safety of anti-CD19 CAR-T cell therapy in R/R PMBL patients and investigate factors associated with treatment outcomes[3].
Methods
This study included all patients with histologically confirmed PMBL who received anti-CD19 CAR-T cell therapy in France between October 2018 and March 2023, and were included in the national DESCAR-T registry (NCT04328298) and analyzed. Baseline patient characteristics, bridging treatments, toxicities, response rates, PFS, and overall survival (OS) were analyzed[3].
Results
Baseline Patient Characteristics
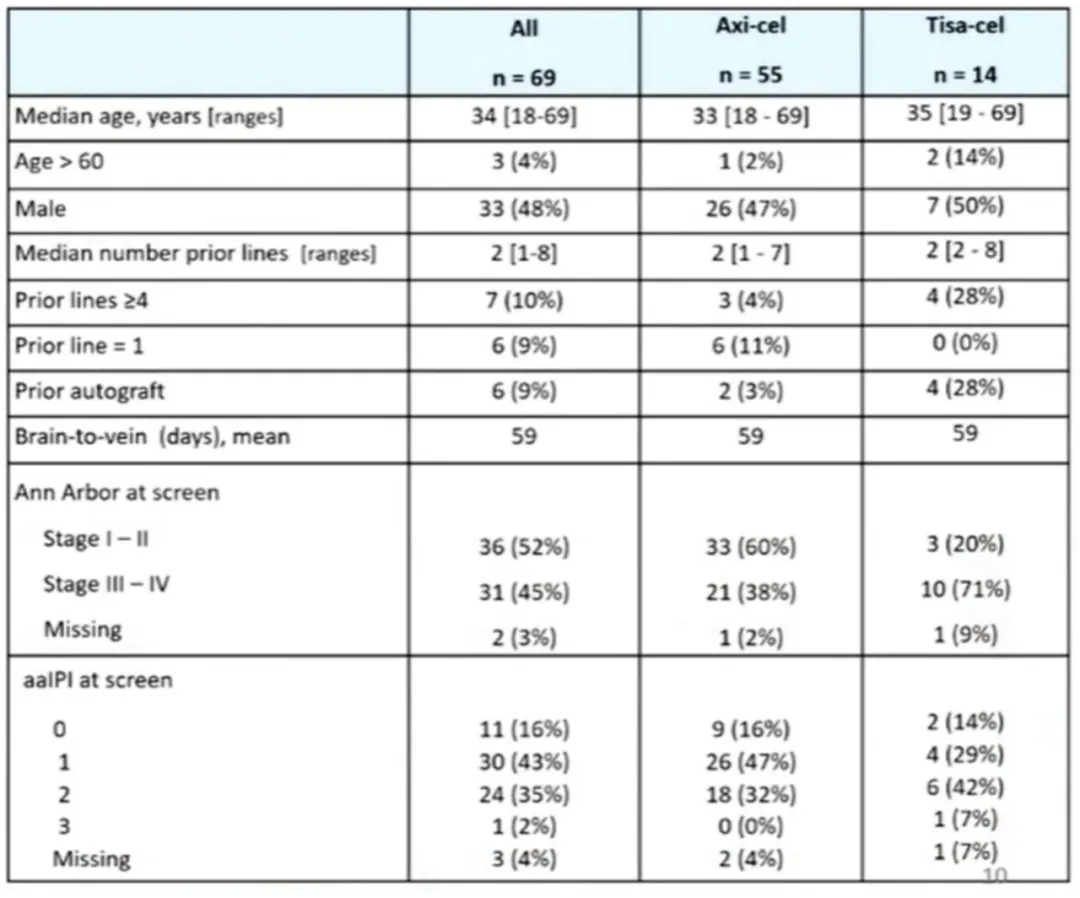
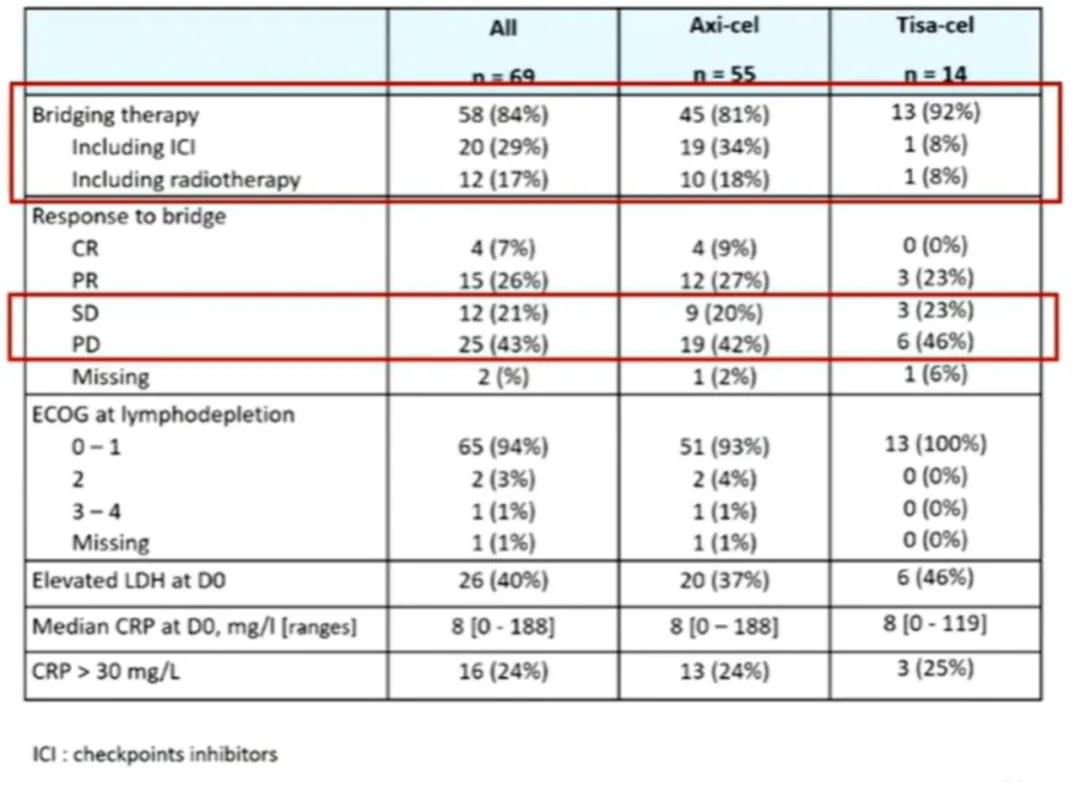
69 R/R PMBL patients received Yescarta (n=55) or Tisagenlecleucel (Tisa-cel) (n=14) treatment. The median follow-up time was 20 months. The median age was 34 years (with 3 patients older than 60 years). The sex ratio (male/female) was 0.9/1. The median number of prior lines of therapy was 2, with 6/69 patients receiving one line (including upfront ASCT) and 7/69 patients receiving ≥4 lines. At the time of treatment decision, the median stage was II, and the median aaIPI score was 1. 58 (84%) patients received bridging therapy, including 12 (17%) who received radiotherapy and 20 (29%) who received immune checkpoint inhibitors (ICIs) (Table 1).
Table 1: Patient Characteristics
Efficacy Results
Among patients who received Yescarta (n=55), the best overall response rate (bORR) was 91%, with CR and partial response (PR) rates of 80% and 11%, respectively. With a median follow-up of 23 months, the 2-year PFS rate was 68.7% [95% CI: 57.5%-82%], and the median PFS was not reached (Figure 1). The 2-year OS rate was 86% [95% CI: 76.2%-96.5%], and the median OS was not reached (Figure 2). For patients achieving CR (n=44), the 2-year PFS rate was 86% [95% CI: 71%-93.5%] (Figure 3).
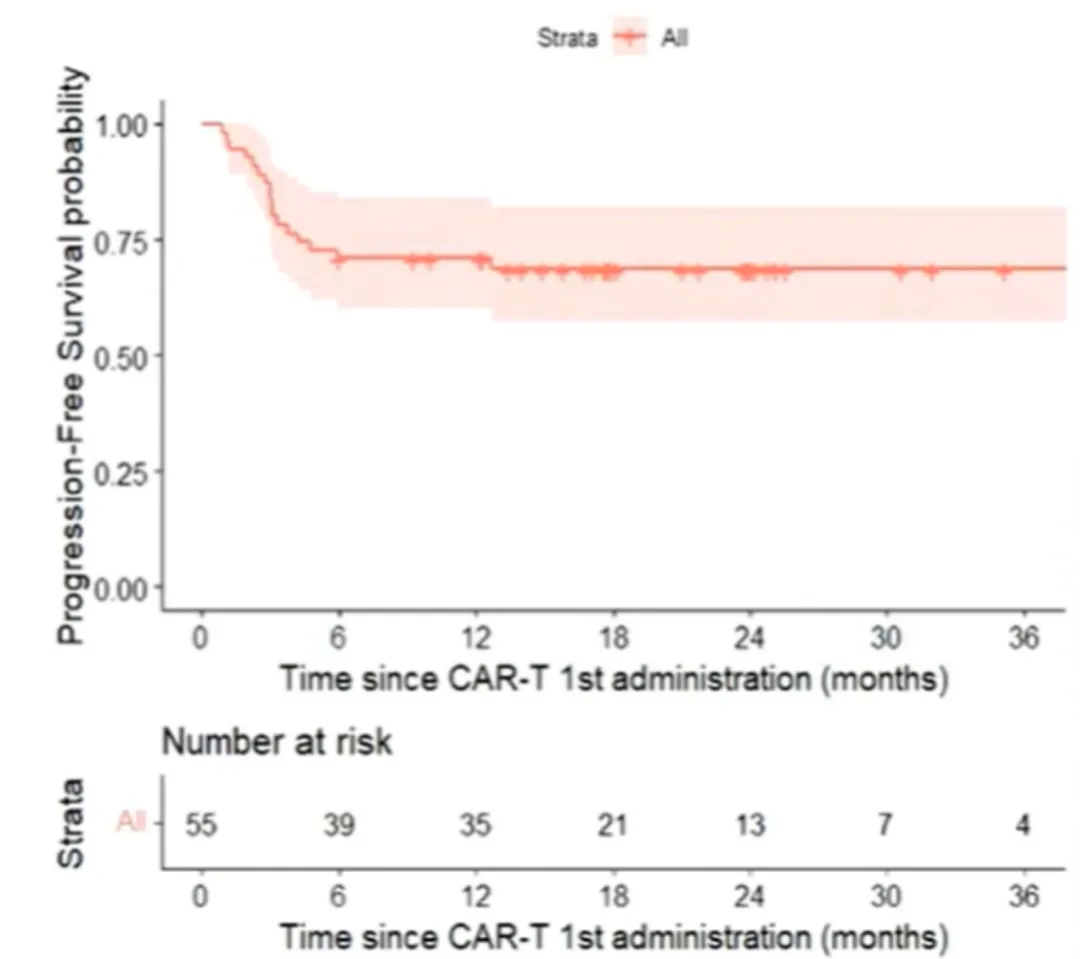
Figure 1: PFS from First CAR-T Treatment
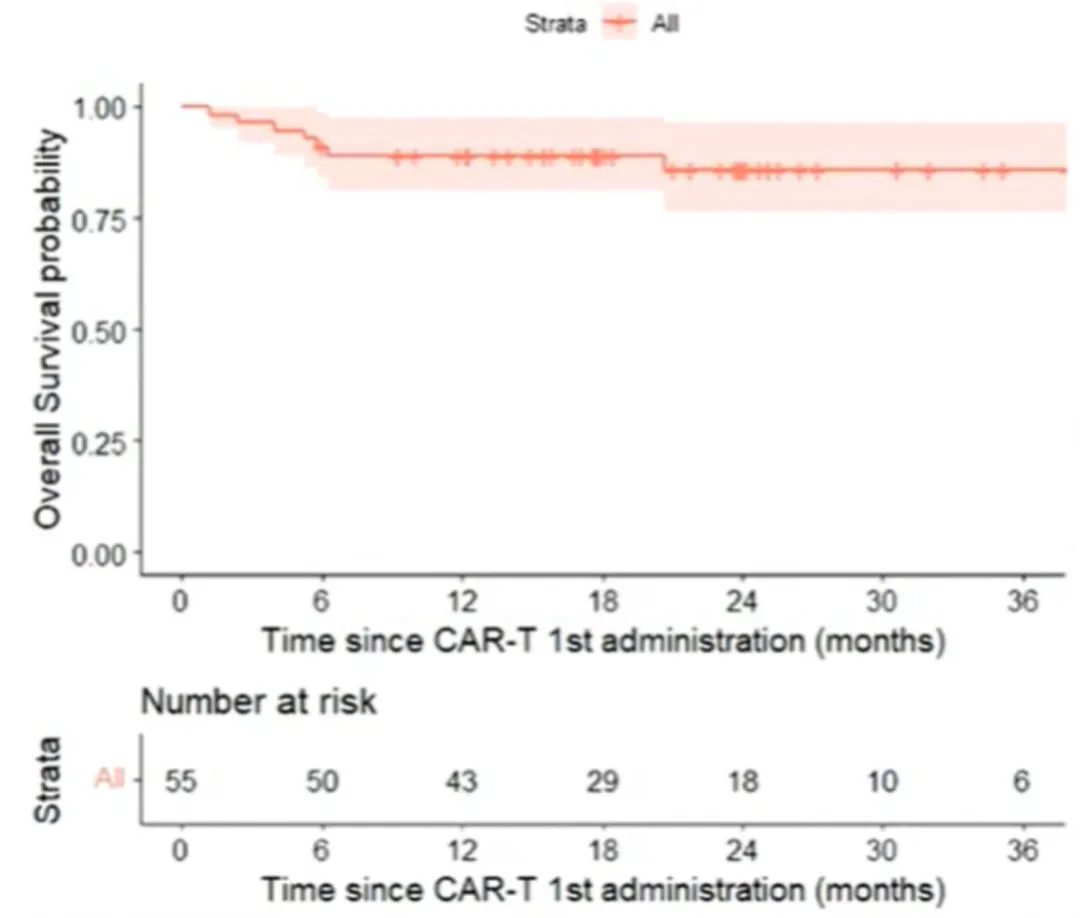
Figure 2: OS from First CAR-T Treatment
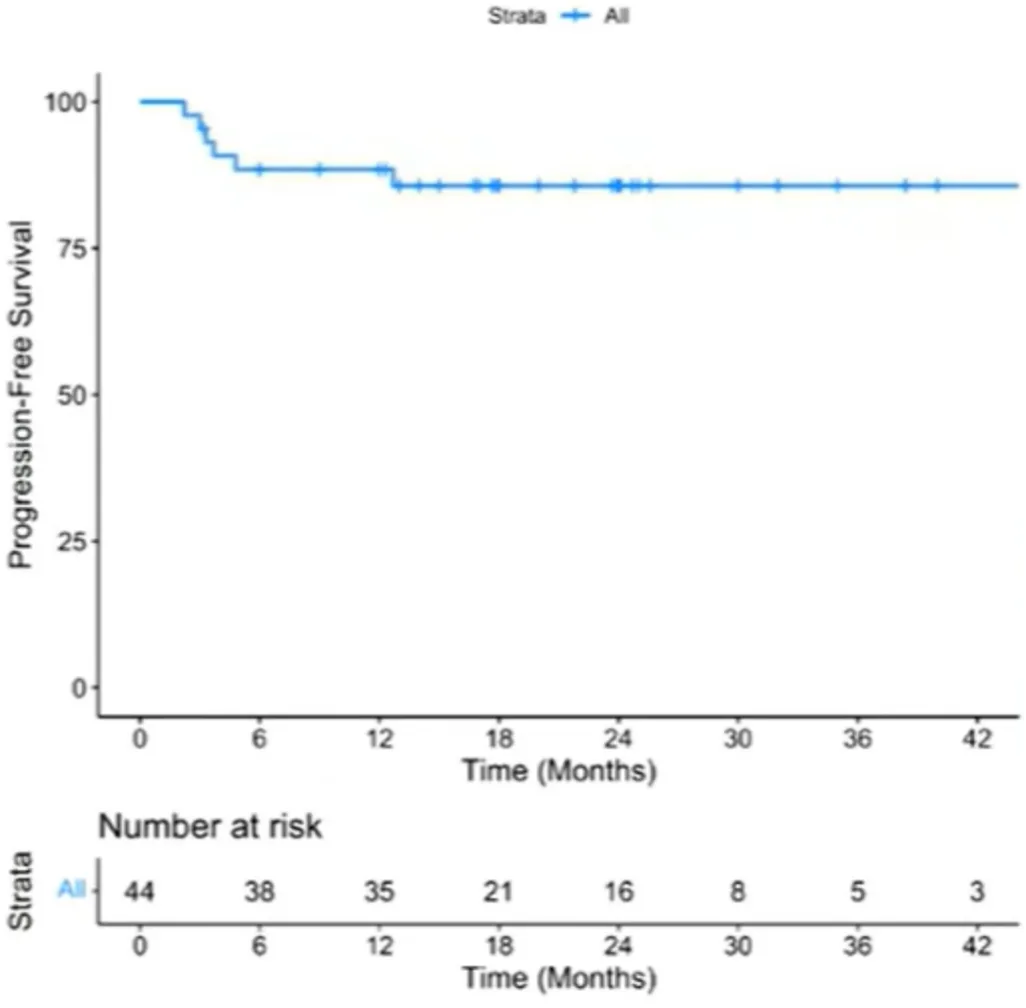
Figure 3: PFS for CR Patients
Factors Associated with Efficacy
In univariate analysis, baseline C-reactive protein (CRP) >30 mg/L was associated with lower PFS (OR: 3.1; p=0.09), while baseline lactate dehydrogenase (LDH) level and response status at bridging were not associated with PFS (Figure 4). Among patients receiving bridging therapy, the bridging regimen with immune checkpoint inhibitors (ICIs) was not associated with better PFS (P=0.61) (Figure 5).
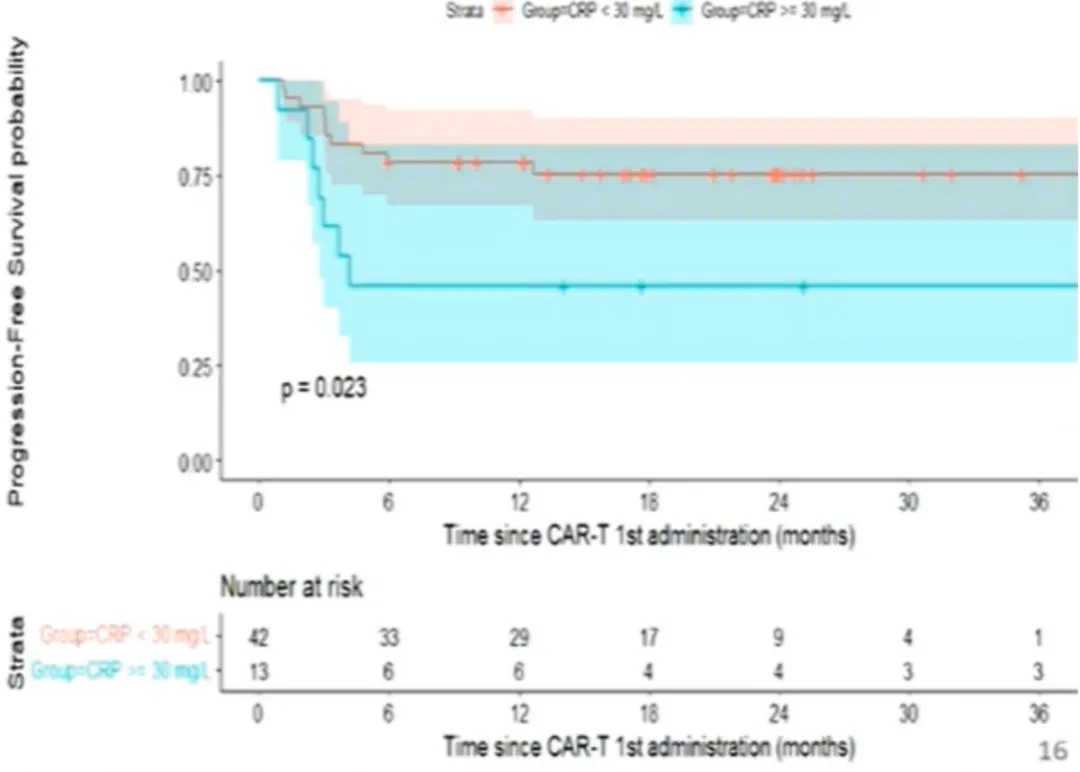
Figure 4: PFS from First CAR-T Treatment (Stratified by CRP)
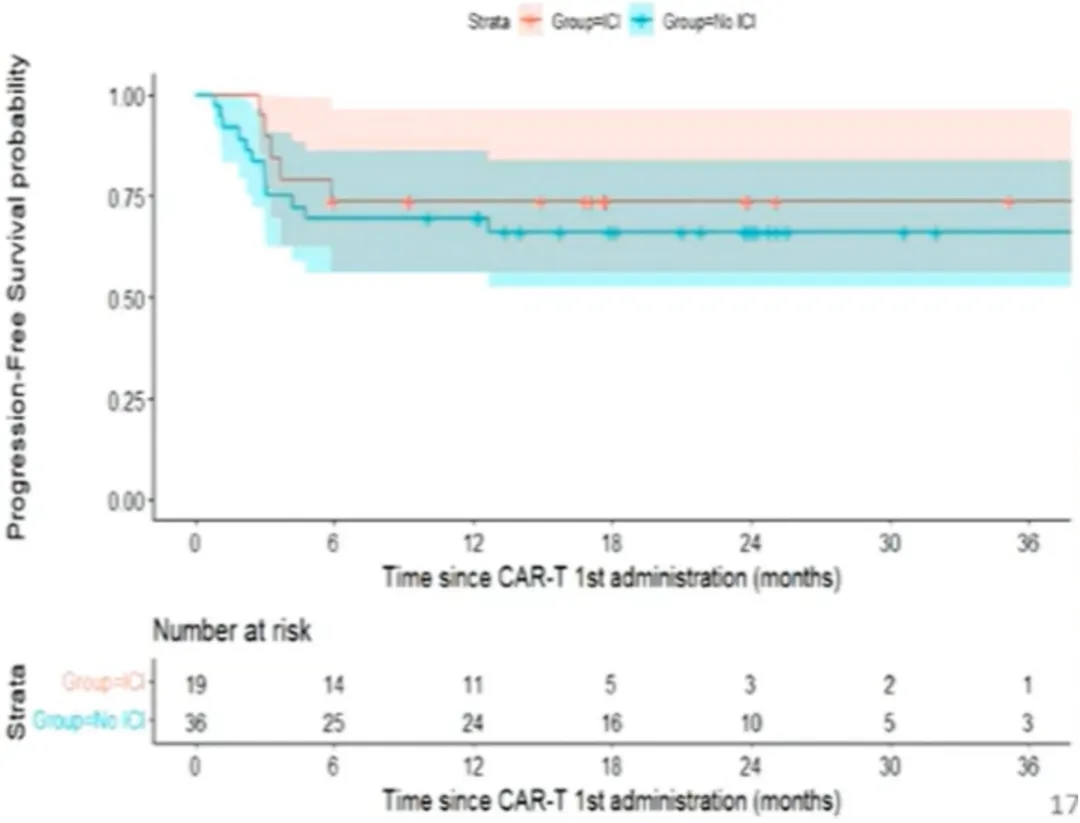
Figure 5: PFS from First CAR-T Treatment (Stratified by ICI)
For patients who relapsed after Yescarta treatment (n=16), the 2-year OS rate was 59% [95% CI: 30%-80%], and the median OS was not reached (Figure 6).
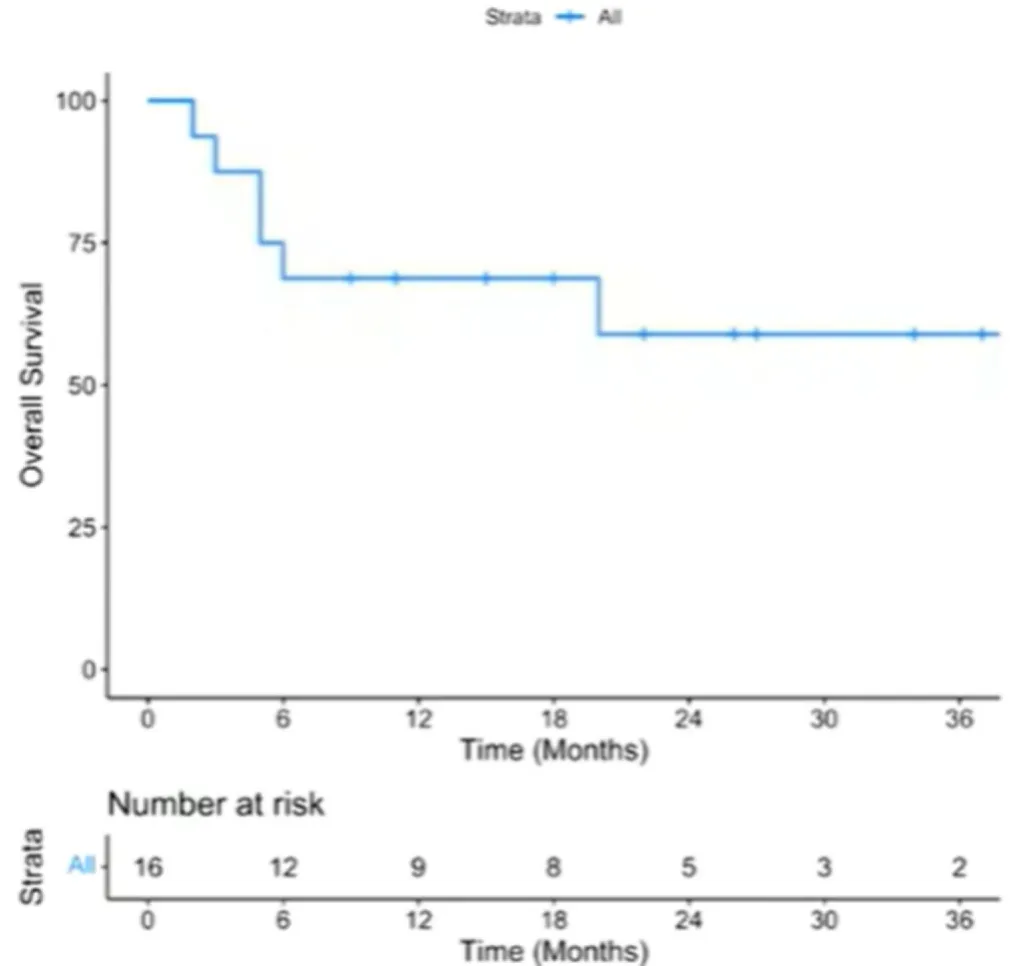
Figure 6: Outcomes After Relapse Post Yescarta
Safety Results
The incidence of any-grade cytokine release syndrome (CRS) was 87%, with 2% grade 3-4 CRS. The incidence of any-grade immune effector cell-associated neurotoxicity syndrome (ICANS) was 43%, with 27% grade 3-4 ICANS. One patient (2%) died due to treatment-related toxicity.
Conclusions
Yescarta demonstrated excellent efficacy in treating R/R PMBL patients. Response status at bridging and bridging therapy type were not associated with treatment outcomes. Patients who relapsed after Yescarta had excellent survival, suggesting an evolving treatment paradigm for PMBL.
References
[1].https://ehaweb.org/congress/eha2024-hybrid-congress/eha2024-hybrid-congress/
[2].Jean Galtier, et al. 2024 EHA, Oral Presentation, S240.
[3].Jean Galtier, et al. 2024 EHA, Abstract, S240
[4].中华医学会血液学分会淋巴细胞疾病学组,中国临床肿瘤学会(CSCO)淋巴瘤专家委员会. 原发纵隔大B细胞淋巴瘤诊断与治疗中国专家共识(2024年版)[J]. 中华血液学杂志,2024,45(03):209-214.
[5].Vardhana S, et al. Outcomes of Relapsed and Refractory Primary Mediastinal (Thymic) Large B Cell Lymphoma Treated with Second-Line Therapy and Intent to Transplant. Biol Blood Marrow Transplant. 2018 Oct;24(10):2133-2138.
Content Source:复星凯特
