CAR-T Cells B Cell Lymphoma Elderly Patients Can Also Benefit
CAR-T Cells B Cell Lymphoma Elderly Patients Can Also Benefit
Diffuse large B-cell lymphoma (DLBCL) is the most common type of aggressive lymphoma, with a median age at diagnosis of 66 years, and approximately 30% of newly diagnosed DLBCL patients are aged ≥75 years1. Age is a major determinant of prognosis in DLBCL, significantly impacting relapse and disease-related mortality rates[2-3].
Based on two pivotal phase 3 studies, CD19 CAR-T cell therapy has become the standard treatment for relapsed/refractory diffuse large B-cell lymphoma (r/r DLBCL)[4-5]. Although the benefits of CAR-T cell therapy are clear in the general patient population, real-world evidence on its efficacy and toxicity in elderly and ECOG PS impaired patients remains relatively scarce.
The American Journal of Hematology and Hemasphere recently published articles investigating the application of CAR-T in elderly DLBCL patients, one comparing CAR-T with immunochemotherapy in the elderly subgroup, and the other comparing CAR-T treatment in patients <70 years and ≥70 years.
Axi-cel superior to immunochemotherapy in elderly and/or ECOG PS impaired patients with ≥2 prior lines of therapy[6]

This study compared the efficacy and effectiveness of Axicabtagene ciloleucel (axi-cel) with immunochemotherapy (CIT) in patients with R/R LBCL who had received ≥2 prior lines of therapy. It included 1146 patients with R/R LBCL who received axi-cel and had received ≥2 prior lines of therapy from the prospective observational study at the Center for International Blood and Marrow Transplant Research, and 469 patients with R/R LBCL who received CIT and had received ≥2 prior lines of therapy from the SCHOLAR-1 study (an international, multi-cohort, retrospective study).
Due to substantial baseline differences between patients, propensity score matching was performed. After propensity score matching, 493 patients in the axi-cel group and 289 patients in the CIT group were included in the response rate analysis set, and 659 patients in the axi-cel group and 406 patients in the CIT group were included in the survival analysis set.
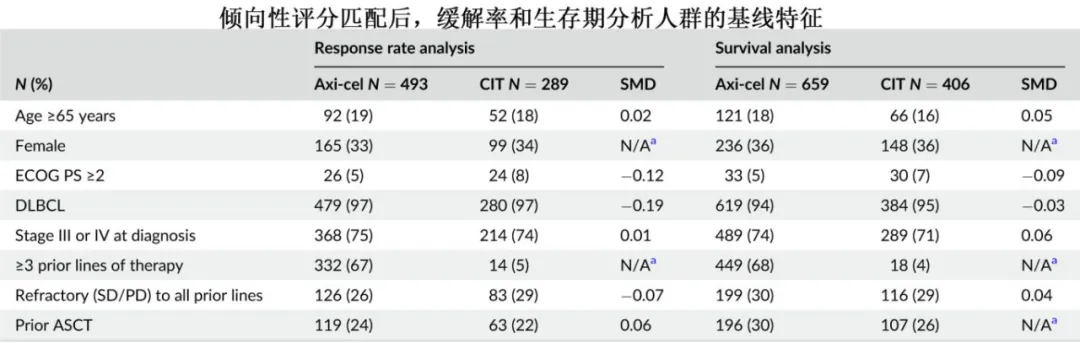
With a median follow-up of 24 months, the 12-month overall survival (OS) rates were 62% in the axi-cel group and 28% in the CIT group (HR=0.30).
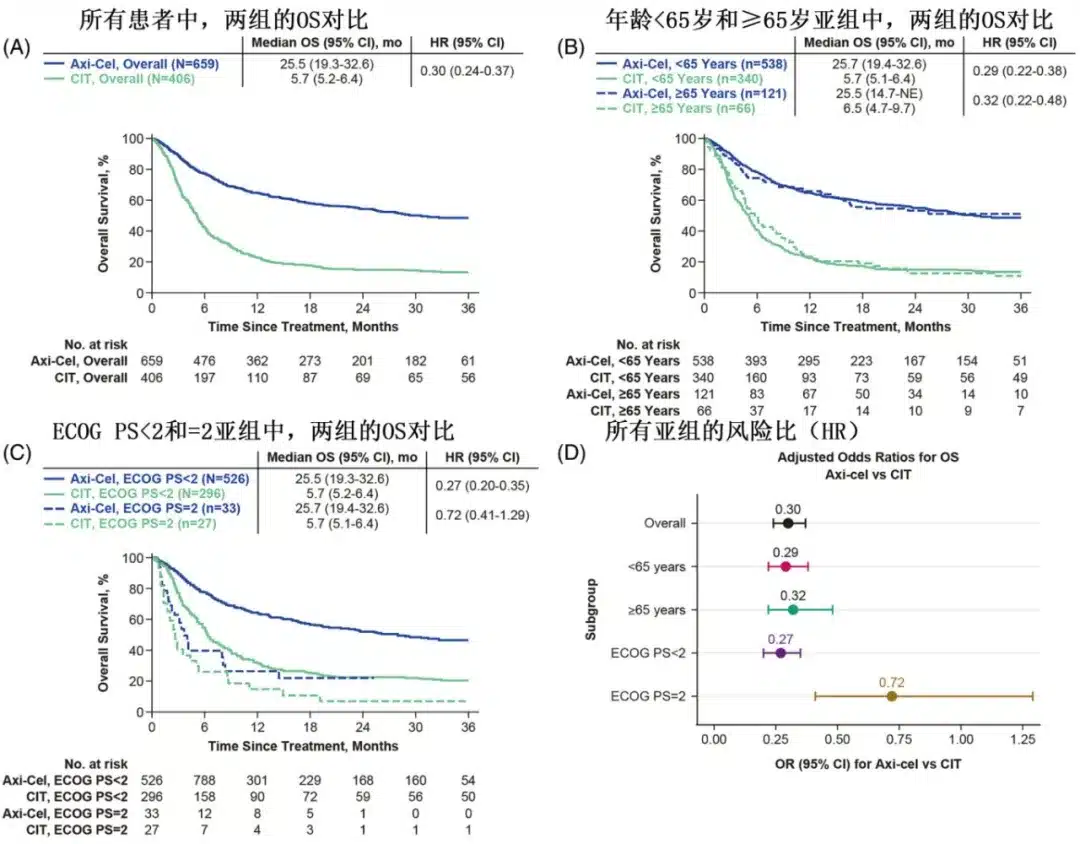
Among patients treated with axi-cel and those treated with CIT, the objective response rates (ORR) were 76% (complete response [CR] rate of 58%) and 28% (CR rate of 16%), respectively; multivariate analysis showed that the axi-cel group had significantly higher ORR (OR=7.73) and CR rates (OR=6.07) compared to the CIT group.
Subgroup analysis by age showed that in patients aged ≥65 years, the difference in ORR between the axi-cel and CIT groups was 57% (82% vs. 25%), and the difference in CR rate was 55% (68% vs. 13%); in patients aged <65 years, the differences in ORR and CR were 46% and 39%, respectively; and the benefit of axi-cel relative to CIT in terms of ORR was numerically greater in patients aged ≥65 years (OR=18.11) than in those aged <65 years (OR=7.14). Compared with the CIT group, the axi-cel group had longer OS in both patients aged ≥65 years (HR=0.32) and those aged <65 years (HR=0.29).
Subgroup analysis by ECOG PS showed that in patients with ECOG PS <2, the difference in ORR between the axi-cel and CIT groups was 52% (62% vs. 10%), and the difference in CR rate was 30% (35% vs. 5%); in patients with ECOG PS=2, the difference in ORR between axi-cel and CIT was 41% (76% vs. 35%), and the difference in CR rate was 35% (58% vs. 23%); the benefit of axi-cel relative to CIT in terms of ORR was similar in patients with ECOG PS <2 and =2 (OR=5.87 and 4.86, respectively). In patients with ECOG PS <2, the axi-cel group had longer OS than the CIT group (HR=0.27), while in patients with ECOG PS=2, there was a numerical difference, but it did not reach statistical significance (HR=0.72).
Summarize
These results indicate that in real-world patients with R/R LBCL, axi-cel treatment provides clinical benefits beyond CIT. In patients aged ≥65 years, the magnitude of response and survival benefit with axi-cel treatment was numerically greater than in the overall population compared to CIT, suggesting that this subgroup may particularly benefit from axi-cel treatment. Clinical benefit of axi-cel over CIT was also observed in patients with ECOG PS=2 (another difficult-to-treat population). Collectively, these data support the use of axi-cel as an effective treatment option for elderly and/or ECOG PS impaired patients with R/R LBCL.
Similar efficacy and safety of CAR-T cell therapy in patients aged ≥70 years and <70 years[7]

This study was a multicenter retrospective analysis that included 172 patients with r/r DLBCL who received CAR-T cell therapy (axicabtagene ciloleucel or tisagenlecleucel). Patients were grouped based on age at the time of CAR-T infusion: <70 years (n=109, median 61 years) and ≥70 years (n=63, median 74 years). Most baseline characteristics were similar between the two groups, but the <70 years group had lower proportions of patients with severe chronic kidney disease (CKD stage 3-4: 10.1% vs. 28.6%, p=0.005), congestive heart failure (NYHA class I-IV: 6.4% vs. 17.5%, p=0.04), atrial fibrillation (7.3% vs. 25.4%, p=0.002), obstructive lung disease (COPD: 0.9% vs. 7.9%, p=0.03), and diabetes (7.3% vs. 19.0%, p=0.03). Additionally, prior auto-HSCT was more common in younger patients (43.1% vs. 14.3%, p<0.001).
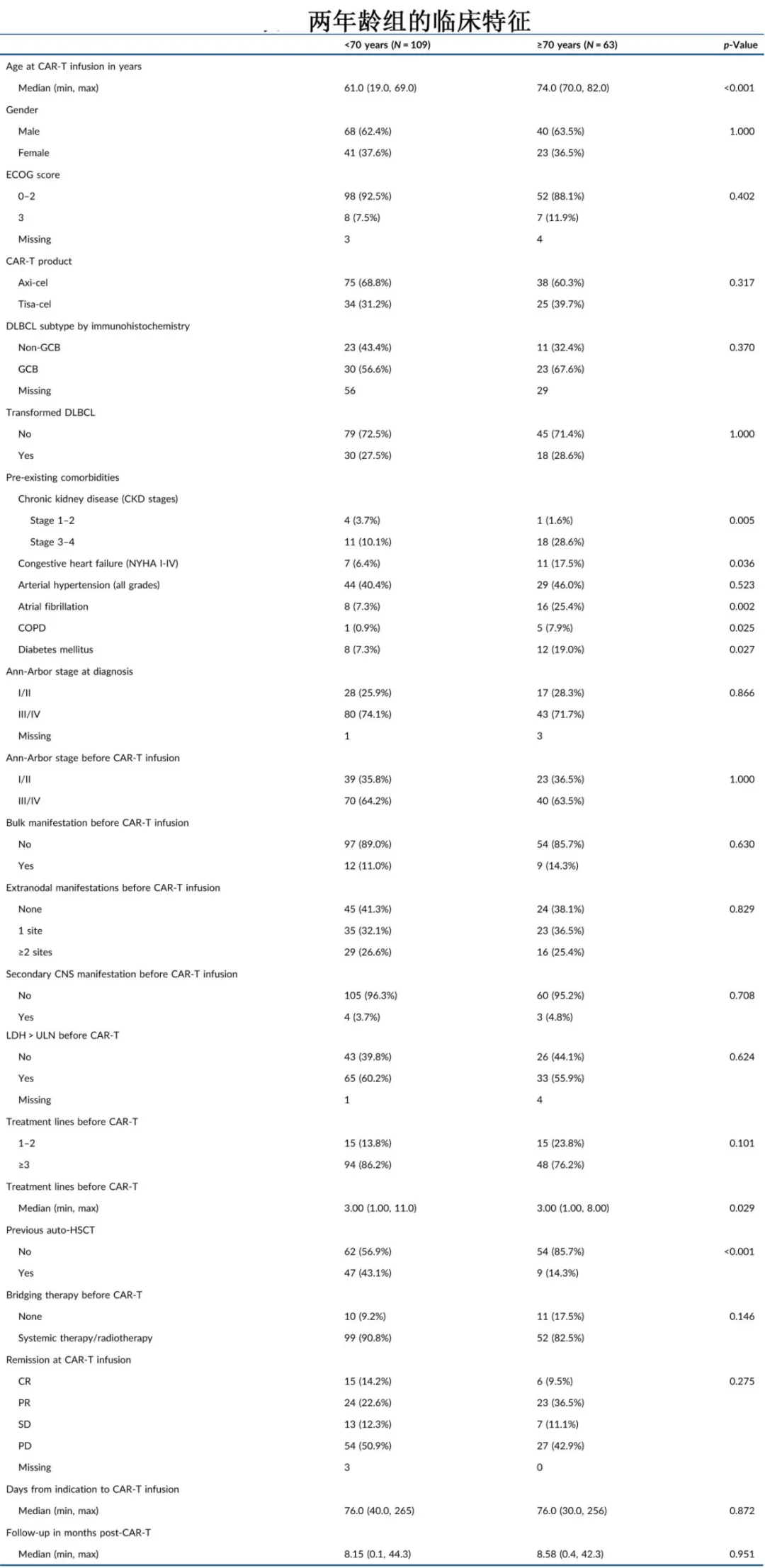
The overall response rates were comparable between the two age groups (77.7% vs. 78.3%; P=0.63), with median times to best response of 2.3 and 3 months, respectively.
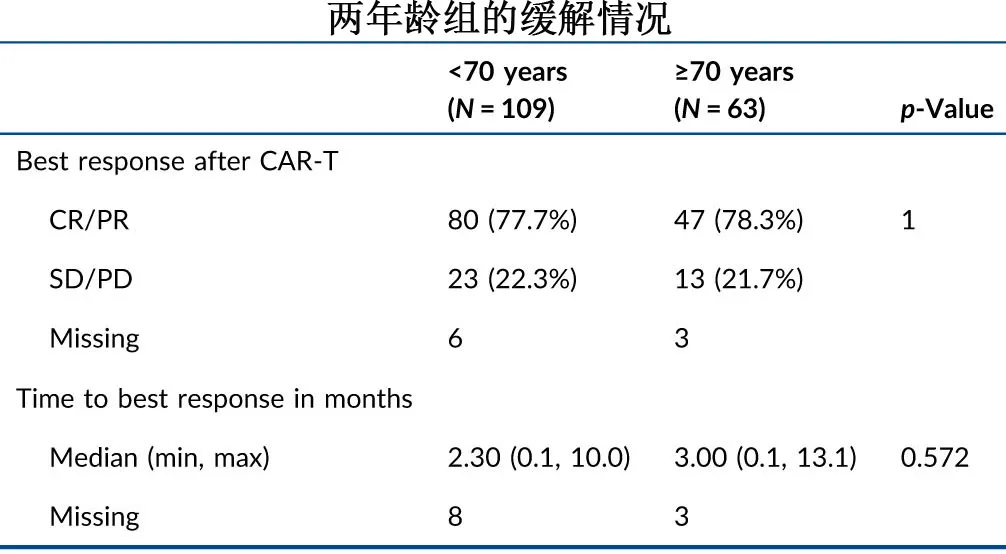
The response patterns before and after CAR-T infusion in the two age groups are shown in the following figure. For patients with progressive disease (PD) at the time of CAR-T therapy, the proportions achieving CR or PR after CAR-T were 72.2% (39/54) in the <70 years group and 77.8% (21/27) in the ≥70 years group.
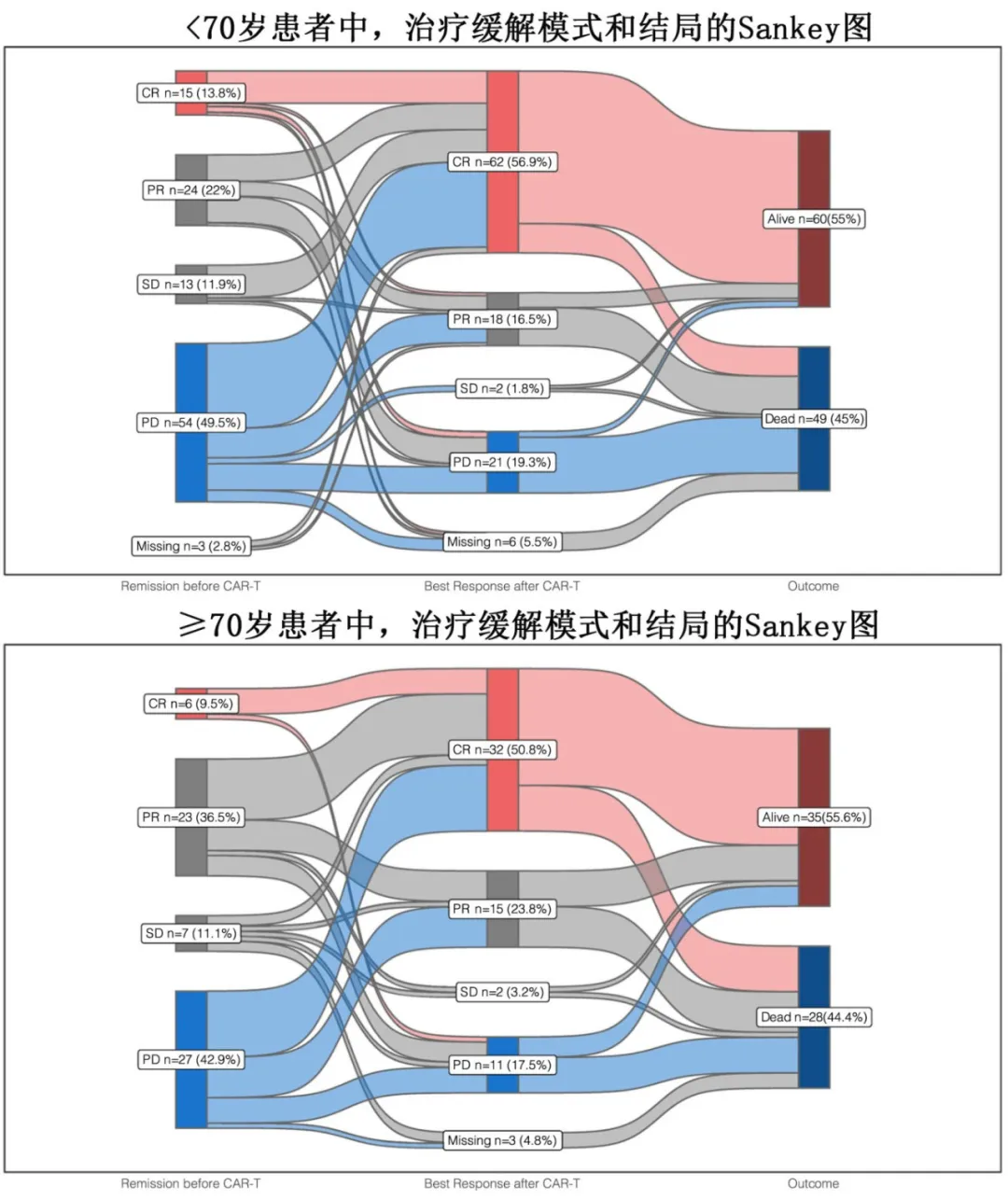
The 3-month relapse rates were 21% in the <70 years group and 12% in the ≥70 years group, and the 1-year relapse rates were 42% and 32%, respectively (p=0.23). With a median follow-up of 8.3 months, the median progression-free survival (PFS) was 10.2 months in the <70 years group and 11.1 months in the ≥70 years group (p=0.93), with 1-year PFS rates of 44.7% and 46.7%, and 2-year PFS rates of 35.5% and 43.4%, respectively; the median overall survival (OS) was 21.8 months in the <70 years group and 34.4 months in the ≥70 years group (p=0.97), with 1-year OS rates of 58.3% and 57.3%, and 2-year OS rates of 47.4% and 51.4%, respectively. Overall, 30 (17.4%) of the 172 patients experienced non-relapse mortality (NRM), primarily due to infections (18/30, 60%) and CRS/ICANS (4/30, 13.3%); of these, 40% (12/30) were early (≤100 days after CAR-T) NRM, and 60% (18/30) were late (>100 days after CAR-T) NRM. Considering relapse/progression as a competing event, the cumulative incidence of NRM was numerically higher in the ≥70 years group compared to the <70 years group, with rates of 14.7% and 8.3% at 3 months, and 23% and 14% at 12 months, respectively (p=0.20).
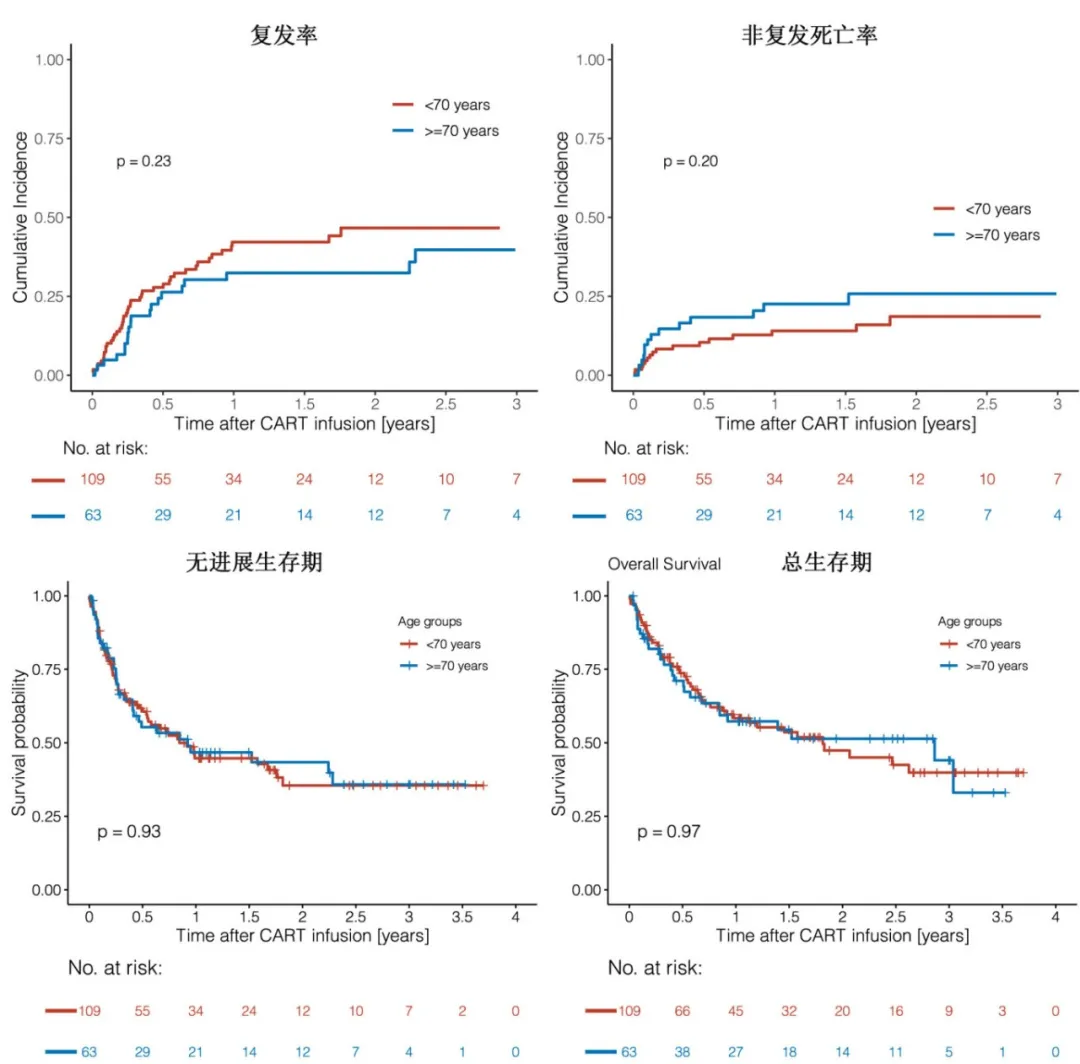
The incidence rates of grade ≥3 cytokine release syndrome (11.9% vs. 7.9%, p=0.45) or grade ≥3 neurotoxicity (18.4% vs. 22.2%, P=0.57) were not significantly different between the <70 years and ≥70 years groups.
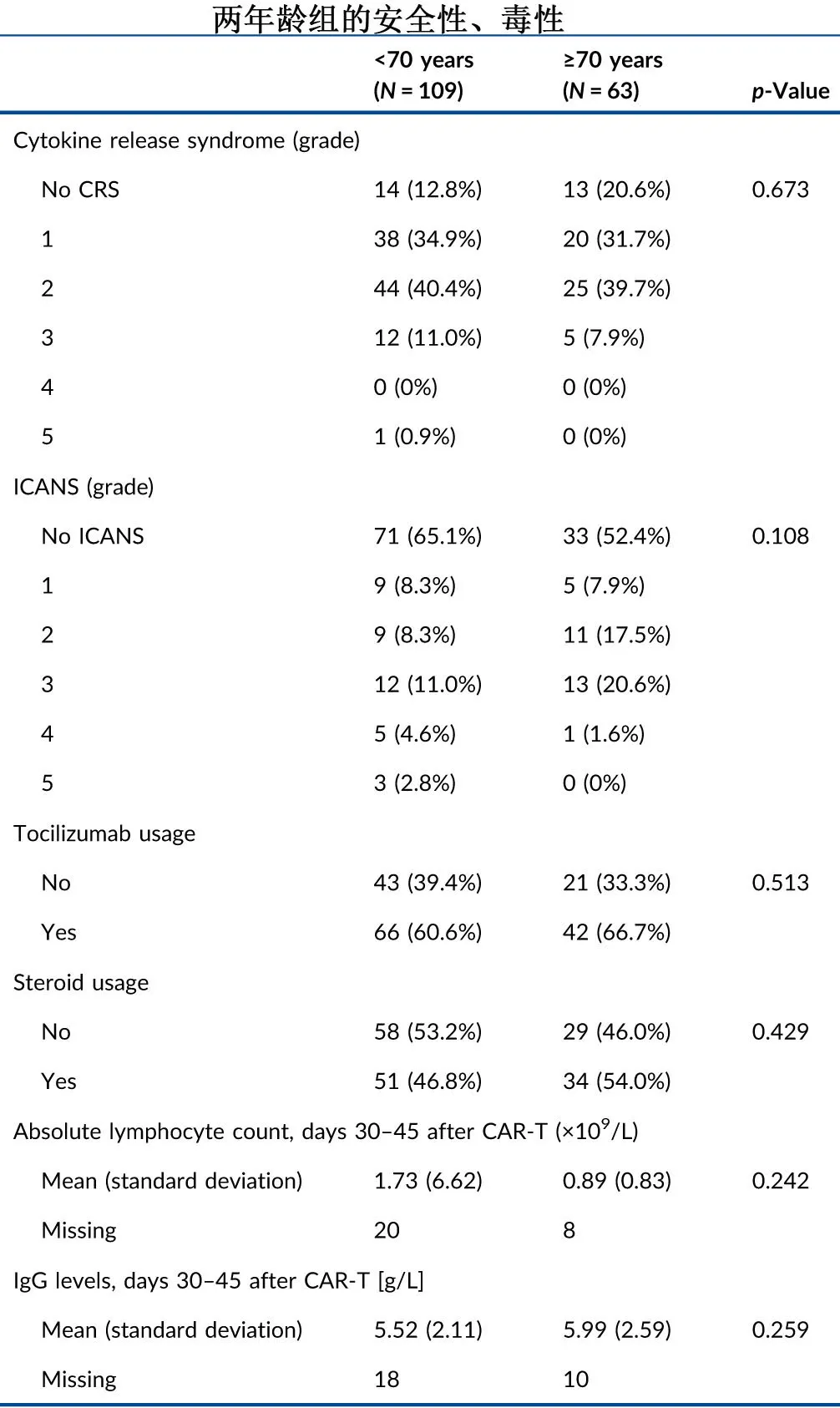
At the last follow-up, 49 (44.9%) and 28 (44.4%) patients had died in the <70 years and ≥70 years groups, respectively, with disease relapse/progression being the leading cause of death, accounting for 33 (67.3%) and 14 (50.0%) cases, respectively. Non-relapse mortality rates were 14.7% and 22.2% in the two groups, respectively, primarily due to grade 5 infections (9.2% and 12.7%), as shown in the table below.
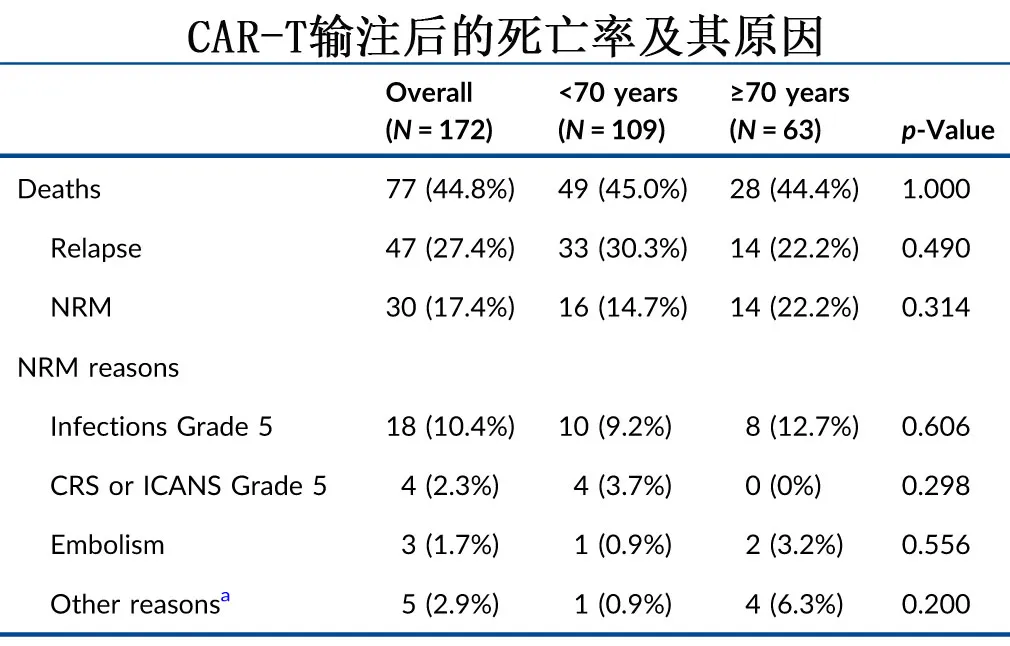
To exclude bias, the two age groups were 1:1 propensity score matched (PSM), and the results showed similar toxicity, response rates, PFS (P=0.47), OS (P=0.48), relapse rates (P=0.51), and NRM (P=0.93).
Summary
These study results further support the feasibility and efficacy of CAR-T cell therapy in patients aged ≥70 years with r/r DLBCL, with outcomes comparable to those of younger patients. However, infection complications and NRM after CAR-T cell therapy should be monitored, particularly in elderly patients. For elderly patients with r/r DLBCL, CAR-T cell therapy should not be denied solely based on age, but longer follow-up studies are needed to further evaluate the long-term safety and efficacy of CAR-T cell therapy in elderly DLBCL patients.
References
1.National Institutes of Health. Cancer Stat Facts: NHL—Diffuse Large B‐cell Lymphoma (DLBCL). NIC; 2020.
2.Kühnl A, Cunningham D, Counsell N, et al. Outcome of elderly patients with diffuse large B‐cell lymphoma treated with R‐CHOP: results from the UK NCRI R‐CHOP14v21 trial with combined analysis of molecular characteristics with the DSHNHL RICOVER‐ 60 trial. Ann Oncol. 2017;28(7):1540‐1546. doi:10.1093/annonc/ mdx128
3.Delarue R, Tilly H, Mounier N, et al. Dose‐dense rituximab‐CHOP compared with standard rituximab‐CHOP in elderly patients with diffuse large B‐cell lymphoma (the LNH03‐6B study): a randomised phase 3 trial. Lancet Oncol. 2013;14(6):525‐533. doi:10.1016/ S1470-2045(13)70122-0
4Abramson JS, Solomon SR, Arnason J, et al. Lisocabtagene maraleucel as second‐line therapy for large B‐cell lymphoma: primary analysis of the phase 3 TRANSFORM study. Blood. 2023;141(14): 1675‐1684. doi:10.1182/blood.2022018730
5.Locke FL, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel as second‐line therapy for large B‐cell lymphoma. N Engl J Med.2022;386(7):640‐654. doi:10.1056/NEJMoa2116133
6.Lunning MA, et al.Benefit of axicabtagene ciloleucel versus chemoimmunotherapy in older patients and/or patients with poor ECOG performance status with relapsed or refractory large B-cell lymphoma after 2 or more lines of prior therapy.Am J Hematol . 2024 Mar 19. doi: 10.1002/ajh.27283.
7.Berning P,et al. Chimeric antigen receptor‐T cell therapy shows similar efficacy and toxicity in patients with diffuse large B‐cell lymphoma aged 70 and older compared to younger patients: A multicenter cohort study.Hemasphere . 2024 Mar 20;8(3):e54. doi: 10.1002/hem3.54.
Content Source:聊聊血液
