CAR-T for Follicular Lymphoma: Lisocabtagene Maraleucel Offers Durable Remission
CAR-T for Follicular Lymphoma: Lisocabtagene Maraleucel Offers Durable Remission
Follicular lymphoma is a lymphoproliferative disorder of B-cell origin. It is a type of non-Hodgkin lymphoma, with overexpression of B-cell lymphoma 2 (BCL2) in most patients. Patients with this cancer present with painless lymphadenopathy, elevated serum lactate dehydrogenase, weight loss, night sweats, and fever. Early-stage follicular lymphoma patients receive radiation therapy[1]. Late-stage patients are treated with anti-CD20 (cluster of differentiation 20) monoclonal antibodies, such as rituximab, and chemotherapeutic agents like bendamustine, cyclophosphamide, vincristine, and doxorubicin. Follicular lymphoma patients have a high risk of relapse and a poor prognosis.
On May 15, 2024, the U.S. Food and Drug Administration (FDA) granted accelerated approval for an immunotherapy treatment for follicular lymphoma[2]. Lisocabtagene maraleucel is a chimeric antigen receptor (CAR) T-cell therapy targeting CD19 (cluster of differentiation 19), an antigen present on B cells. CAR T-cell therapy is a novel form of immunotherapy for cancer treatment. It involves genetically modifying a patient’s T cells to recognize cancer cells. After genetic engineering, the T cells express a CAR, a specialized receptor that binds to antigens. These cells are expanded in the laboratory and infused back into the patient.
Lisocabtagene maraleucel, developed by Juno Therapeutics, is administered as a one-time treatment. The FDA had previously approved this therapy for the treatment of large B-cell lymphoma and small lymphocytic lymphoma or chronic lymphocytic leukemia. Lisocabtagene maraleucel is indicated for patients who have received at least two prior lines of therapy, including an alkylating agent and an anti-CD20 antibody. The recommended dose is 90 to 110 x 106 CAR-positive T cells, with a CD8:CD4 component ratio of 1:1. The CAR construct comprises a CD3 zeta signaling domain fused to a 4-1BB co-stimulatory domain, promoting CAR T-cell proliferation. Other components include an IgG4 hinge, a single-chain variable fragment (scFv), and a CD28 transmembrane domain.
The approval was based on the results of the phase 2 TRANSCEND-FL clinical trial, with preliminary data presented at the 2023 International Conference on Malignant Lymphoma[3]. According to the initial analysis, the overall response rate was 97% among 101 evaluable follicular lymphoma patients treated with lisocabtagene maraleucel as third-line or later (3L+) therapy[4]. Complete responses were observed in 94.1% of patients. At a median follow-up of 16.6 months, the median duration of response was not reached. At one year, 81.9% of patients continued to respond to the treatment. The median progression-free survival was also not reached at a median follow-up of 17.5 months, with a one-year progression-free survival rate of 80.7%. In the trial, 130 patients received CAR T-cell therapy as second-line or later (2L+) treatment, with 15% and 58% experiencing neurological events and cytokine release syndrome, respectively.
Additional data from the trial were presented at the 2023 American Society of Hematology (ASH) Annual Meeting and Exposition. Among 23 evaluable second-line follicular lymphoma patients, the overall response rate was 95.7%[5]. At a median follow-up of 16.8 months, the median duration of response was not reached. At one year, 89.8% of patients continued to respond. The median progression-free survival was also not reached at a median follow-up of 17.8 months, with a one-year progression-free survival rate of 91.3%[6]. Cytokine release syndrome and neurological events occurred in 52.2% and 17.4% of patients, respectively.
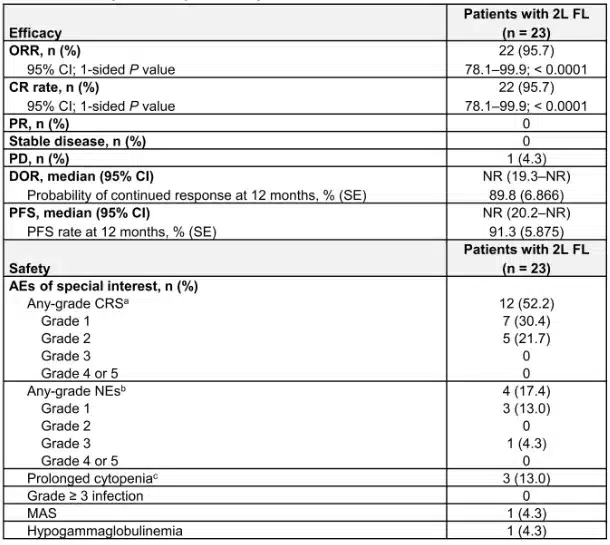
Image 1: Efficacy and safety of lisocabtagene maraleucel [Source: Reference 5]
Previously, axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah) received accelerated FDA approval for the treatment of follicular lymphoma. Axicabtagene ciloleucel is a CD19-targeted CAR T-cell therapy developed by Kite Pharma. In the phase 2 ZUMA-5 trial, the objective response rate was 91% among 81 evaluable follicular lymphoma patients[7]. Complete responses were observed in 60% of patients. The median duration of response was not reached, with 76.2% of patients remaining in response at 12 months. The trial enrolled 127 follicular lymphoma patients and 31 marginal zone lymphoma patients to receive axicabtagene ciloleucel[8]. At a 3-year follow-up, the median duration of response and median progression-free survival for follicular lymphoma patients were 38.6 months and 40.2 months, respectively.
Tisagenlecleucel is a CD19-targeted CAR T-cell therapy developed by Novartis. In the preliminary analysis of the phase 2 ELARA trial, the overall response rate was 86% among 90 evaluable follicular lymphoma patients, with 68% achieving complete responses[9]. The median duration of response was not reached, and 75% of responding patients continued to respond at 9 months. Safety was evaluated in 97 patients, with 53% and 43% experiencing cytokine release syndrome and neurological events, respectively[10]. At a median follow-up of 29 months, the overall response rate was 86.2%[11]. The 2-year overall survival and progression-free survival rates were 87.7% and 57.4%, respectively.
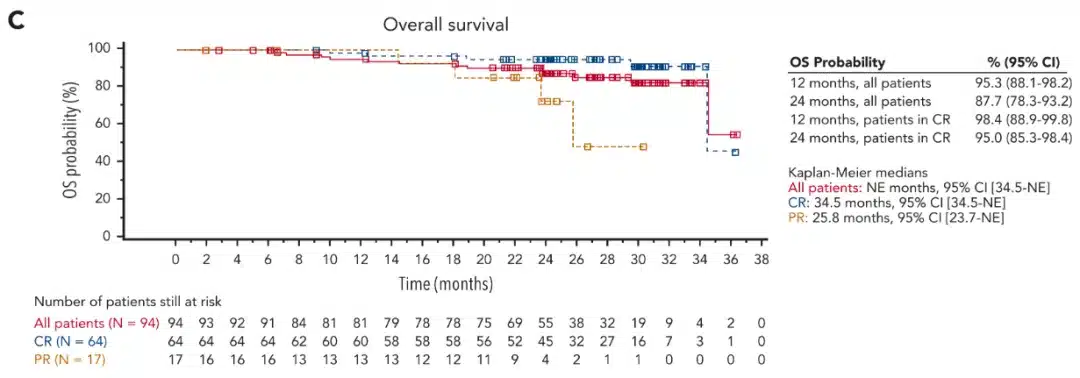
Image 2: Overall Survival of patients treated with tisagenlecleucel [Source: Reference 11]
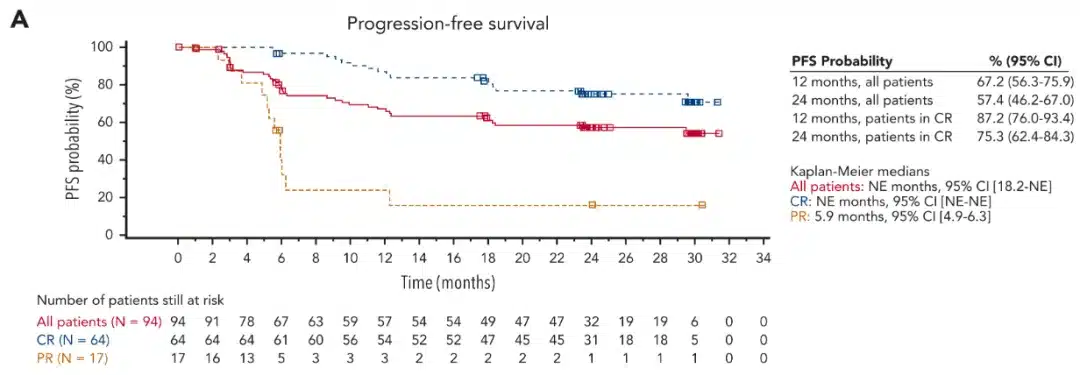
Image 3: Progression-Free Survival of patients treated with tisagenlecleucel [Source: Reference 11]
In summary, lisocabtagene maraleucel has received accelerated FDA approval for the treatment of relapsed or refractory follicular lymphoma. Follicular lymphoma patients treated with this therapy demonstrated durable remissions in the phase 2 clinical trial.
References
[1] Jacobsen, E. (2022). Follicular lymphoma: 2023 update on diagnosis and management. American Journal of Hematology, 97(12), 1638-1651.
[2] FDA (2024, 15 May). FDA grants accelerated approval to lisocabtagene maraleucel for follicular lymphoma. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-lisocabtagene-maraleucel-follicular-lymphoma#:~:text=On%20May%2015%2C%202024%2C%20the,prior%20lines%20of%20systemic%20therapy.
[3] Bristol Myers Squibb (2024, 15 May). Bristol Myers Squibb’s CAR T Cell Therapy Breyanzi Approved by the U.S. Food and Drug Administration for Relapsed or Refractory Follicular Lymphoma. https://news.bms.com/news/corporate-financial/2024/Bristol-Myers-Squibbs-CAR-T-Cell-Therapy-Breyanzi-Approved-by-the-U.S.-Food-and-Drug-Administration-for-Relapsed-or-Refractory-Follicular-Lymphoma/default.aspx
[4] Nastoupil, L. J., Dahiya, S., Palomba, M. L., Garcia-Sancho, A. M., Ortega, J. L. R., Kuruvilla, J., … & Morschhauser, F. (2023). IBCL-098 TRANSCEND FL: phase II study results of lisocabtagene maraleucel (Liso-Cel) in patients with R/R follicular lymphoma (FL). Clinical Lymphoma Myeloma and Leukemia, 23, S447-S448.
[5] Morschhauser, F., et al., (2023, 10 December). 602 TRANSCEND FL: Phase 2 Study Primary Analysis of Lisocabtagene Maraleucel as Second-Line Therapy in Patients with High-Risk Relapsed or Refractory Follicular Lymphoma. https://ash.confex.com/ash/2023/webprogram/Paper179474.html
[6] Bristol Myers Squibb (2023, 10 December). Bristol Myers Squibb Presents New Data at ASH 2023 Demonstrating Clinical Benefit Across B-cell Malignancies with Breyanzi as a Second-Line Treatment in High-Risk Follicular Lymphoma and in Relapsed or Refractory Chronic Lymphocytic Leukemia. https://news.bms.com/news/corporate-financial/2023/Bristol-Myers-Squibb-Presents-New-Data-at-ASH-2023-Demonstrating-Clinical-Benefit-Across-B-cell-Malignancies-with-Breyanzi-as-a-Second-Line-Treatment-in-High-Risk-Follicular-Lymphoma-and-in-Relapsed-or-Refractory-Chronic-Lymphocytic-Leukemia/default.aspx
[7] FDA (2021, 8 March). FDA grants accelerated approval to axicabtagene ciloleucel for relapsed or refractory follicular lymphoma. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-axicabtagene-ciloleucel-relapsed-or-refractory-follicular-lymphoma#:~:text=On%20March%205%2C%202021%2C%20the,more%20lines%20of%20systemic%20therapy.
[8] Neelapu, S. S., Chavez, J., Sehgal, A. R., Epperla, N., Ulrickson, M., Bachy, E., … & Jacobson, C. A. (2022). 3-year follow-up analysis of ZUMA-5: a phase 2 study of axicabtagene ciloleucel (Axi-Cel) in patients with relapsed/refractory (R/R) indolent non-Hodgkin lymphoma (iNHL). Blood, 140(Supplement 1), 10380-10383.
[9] FDA (2022, 31 May). FDA approves tisagenlecleucel for relapsed or refractory follicular lymphoma. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tisagenlecleucel-relapsed-or-refractory-follicular-lymphoma
[10] Novartis (2022, 28 May). FDA approves Novartis Kymriah® CAR-T cell therapy for adult patients with relapsed or refractory follicular lymphoma. https://www.novartis.com/news/media-releases/fda-approves-novartis-kymriah-car-t-cell-therapy-adult-patients-relapsed-or-refractory-follicular-lymphoma
[11] Dreyling, M., Fowler, N. H., Dickinson, M., Martinez-Lopez, J., Kolstad, A., Butler, J., … & Schuster, S. J. (2024). Durable response after tisagenlecleucel in adults with relapsed/refractory follicular lymphoma: ELARA trial update. Blood, 143(17), 1713-1725.
Content Source:肿瘤药闻
