CAR-T for Mantle Cell Lymphoma
CAR-T for Mantle Cell Lymphoma
CAR-T Therapy for MCL
Mantle cell lymphoma (MCL) is a subtype of mature B-cell non-Hodgkin lymphoma (NHL) characterized by a chromosomal translocation, typically resulting in overexpression of Cyclin D1. Despite advances in understanding MCL pathogenesis and treatment strategies, MCL remains incurable. Adverse prognostic markers include older age, male sex, high proliferative rate, bulky lymphadenopathy, elevated serum LDH, and adverse biomarkers (increased Ki-67 expression and TP53 mutations). Advanced immunotherapy using CAR-T cells has benefited B-cell malignancies including MCL patients. Targeting B-cell antigens on the cell surface represents a viable approach for relapsed/refractory (R/R) MCL given significant responses seen in other B-cell cancers. However, major impediments to successful CAR-T cell therapy include severe adverse events, limited anti-tumor activity, antigen escape, limited tumor trafficking, and transportation issues.
Recently, a review was published in Molecular Cancer by corresponding authors Prof. Hongbing Zhao from The First Affiliated Hospital of Xinxiang Medical University and Prof. Vivek P. Chavda from Lallubhai Motilal College of Pharmacy (India), and first authors Prof. Zoufang Huang from The First Affiliated Hospital of Gannan Medical University and Prof. Vivek P. Chavda. The review briefly summarized the development of CAR-T cell therapy for MCL. Key points are compiled here for reference.

Treatment Strategies for MCL
The main treatment strategies for MCL vary based on age and disease characteristics, as summarized in Figure 1. For young and fit patients, intensive immunochemotherapy regimens containing cytarabine can be initiated, followed by autologous stem cell transplant (ASCT). For elderly or unfit patients, less intensive chemotherapy and continuous rituximab represent better alternatives; nanotherapeutic interventions are also being developed.
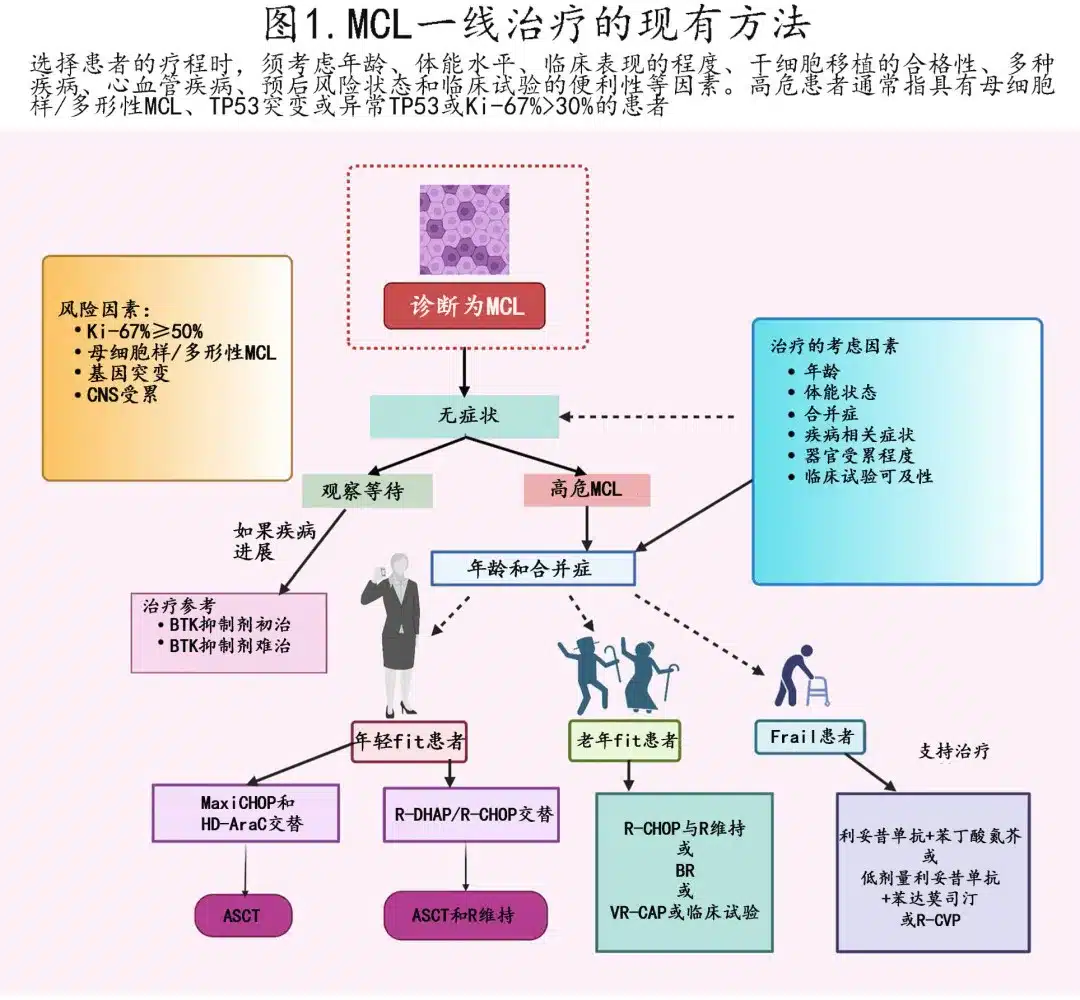
One regimen is the Nordic protocol of maxi-CHOP (high-dose vincristine, doxorubicin, cyclophosphamide, and prednisone) alternating with high-dose cytarabine and rituximab. Another strategy uses R-CHOP alternating with R-DHAP (rituximab, cytarabine, dexamethasone ± platinum). Rituximab and bendamustine (BR) can be combined sequentially or alternated with high-dose cytarabine. Post-ASCT, maintenance rituximab also improves overall survival (OS).
While ASCT at first remission improves progression-free survival (PFS) but not OS, studies have re-evaluated the value of the transplant component. The ongoing ECOG-ACRIN 4151 phase III randomized study is assessing post-transplant rituximab in NGS “MRD-negative” patients to evaluate its performance versus rituximab alone; results suggest most young patients benefit from intensified therapy, but some high-risk groups like high Ki-67 or TP53 mutants do not derive significant advantage.
Older patients or those with comorbidities cannot tolerate intensive cytarabine-containing regimens; BR or rituximab, bortezomib, prednisone, cyclophosphamide, doxorubicin are recommended regimens, while previously untreated patients may be offered rituximab and lenalidomide.
For R/R MCL, immunochemotherapy is less effective than upfront due to frequent relapses and poor prognosis. Targeted agents are typically used due to favorable safety and efficacy. The earliest approved targeted agents were bortezomib, temsirolimus, and lenalidomide, but BTK inhibitors have now emerged as second-line stars. Ibrutinib, acalabrutinib, and zanubrutinib offer short half-lives but convenient dosing. Combination therapy is also being widely explored in R/R MCL, with ibrutinib plus venetoclax showing striking efficacy superior to either agent alone while maintaining tolerable safety, achieving significant response rates of 42% (PET-negative) and 62% (PET-positive) complete response (CR) rates by week 16. The phase III SYMPATICO trial is analyzing the efficacy of ibrutinib plus venetoclax versus ibrutinib alone in R/R MCL.
Effective treatment for relapse after BTKi therapy represents an urgent clinical need, and while the optimal sequence post-BTKi failure is unclear, options include venetoclax, chemoimmunotherapy, and immunotherapy. A retrospective analysis showed that rituximab, bendamustine, and cytarabine (R-BAC) yielded a high ORR (83%) among MCL survivors achieving objective response after BTKi therapy, although response durations were short (PFS 10.1 months); R-BAC has been used as a bridge to ASCT consolidation for transplant-eligible patients.
Additionally, BCL2 inhibitors may have utility in MCL given BCL2 is frequently overexpressed. Venetoclax is a potent selective BCL2 inhibitor, achieving a 75% response rate (21% CR) among 28 R/R MCL patients in a phase I study. While BTK and BCL2 inhibitors offer high response rates, their use is limited by expanding resistance. Evidence suggests BCL2 family mutations underlie acquired venetoclax resistance in MCL.
One study evaluated allo-SCT as salvage and upfront therapy for R/R and MCL patients, respectively, showing comparable outcomes with 5-year OS of 73%. Per Simon Rule et al., early allo-SCT may benefit higher-risk younger adults, particularly TP53 mutant patients.
Conventional therapies have limitations, and the development of anti-CD19 CAR-T cells has transformed treatment for R/R lymphoid malignancies, with CAR-T cell adoptive immunotherapy providing effective and durable responses in BCL2-inhibitor subgroups. Based on the ZUMA-2 study, CAR-T cell therapy represents the most efficacious treatment for MCL and R/R MCL compared to other options.
CAR-T Cell Therapy
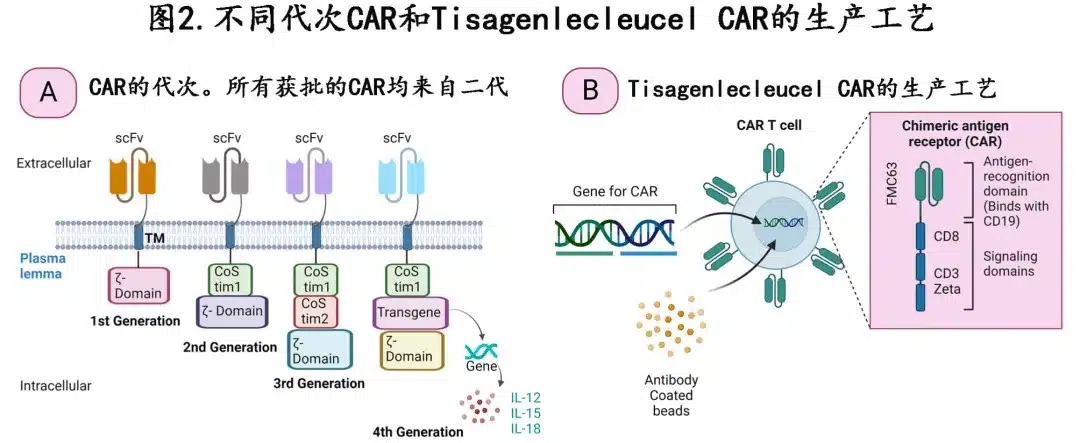
Figure 2A depicts different CAR generations
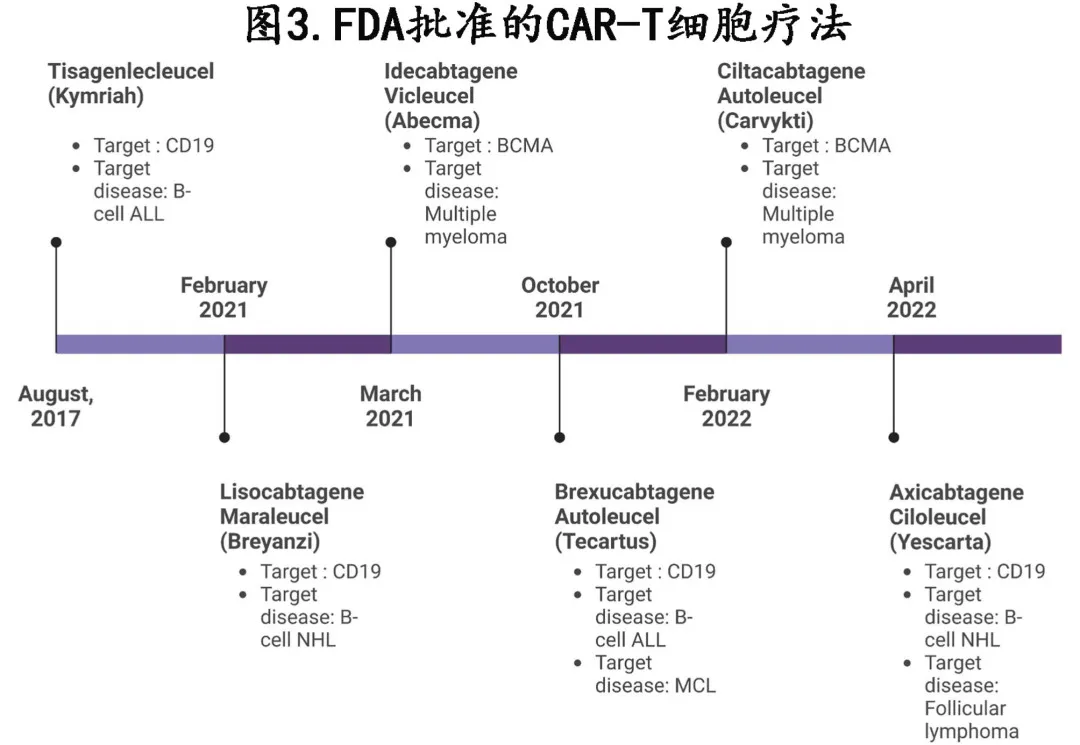
To date, the FDA has approved 6 CAR-T cell therapies for various hematologic malignancies including multiple myeloma and leukemia (Figure 3) since 2017: axicabtagene ciloleucel, tisagenlecleucel, idecabtagene vicleucel, ciltacabtagene autoleucel, lisocabtagene maraleucel and brexucabtagene autoleucel, with the CD19-targeting brexucabtagene autoleucel being most effective for MCL.
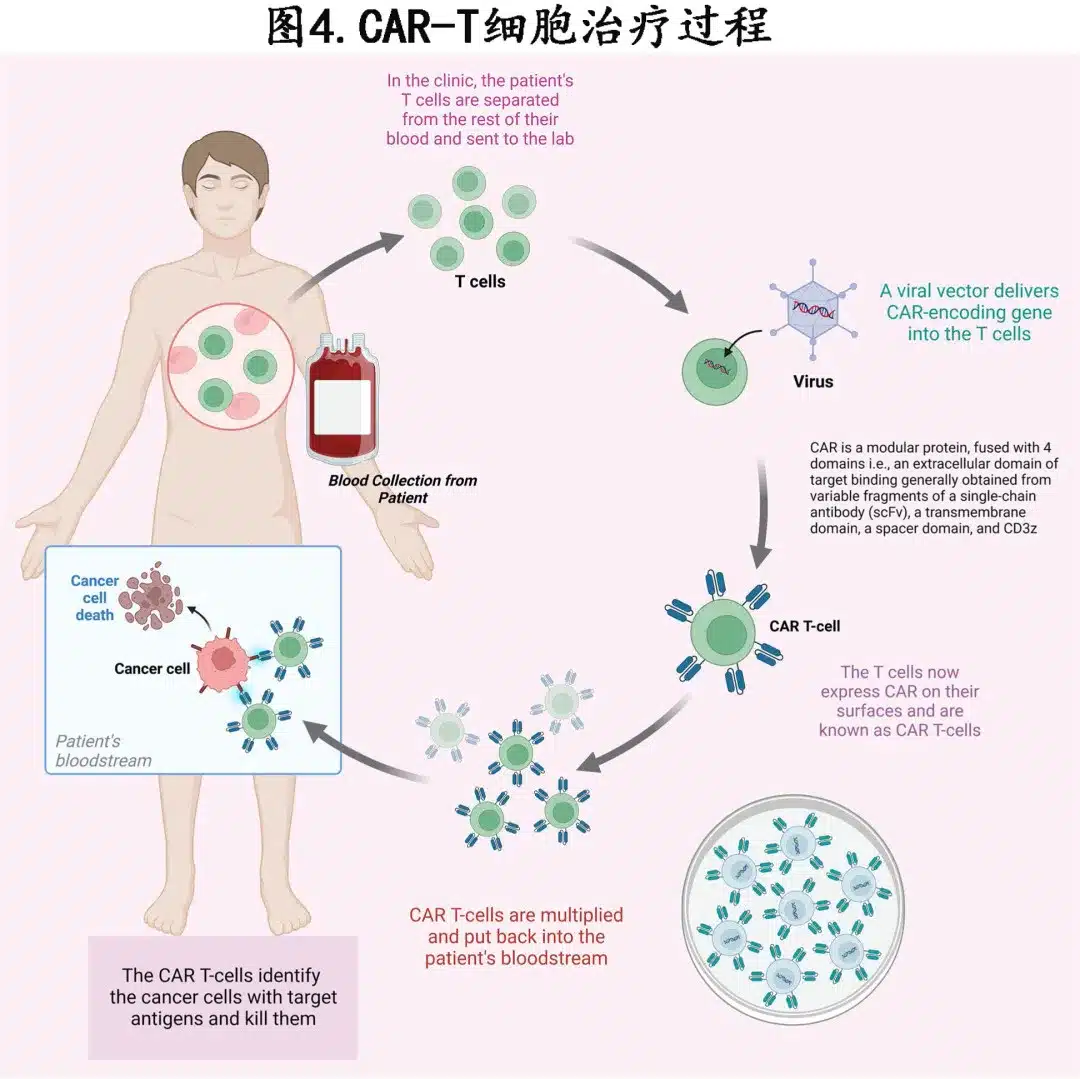
Figures 2B and 4 describe the CAR-T cell manufacturing process.
CAR-T Cell Use in MCL
The ZUMA-2 and TRANSCEND NHL 001 studies achieved promising results in relapsed/refractory MCL patients. Table 1 summarizes various clinical developments and outcomes with CAR-T cells in MCL.
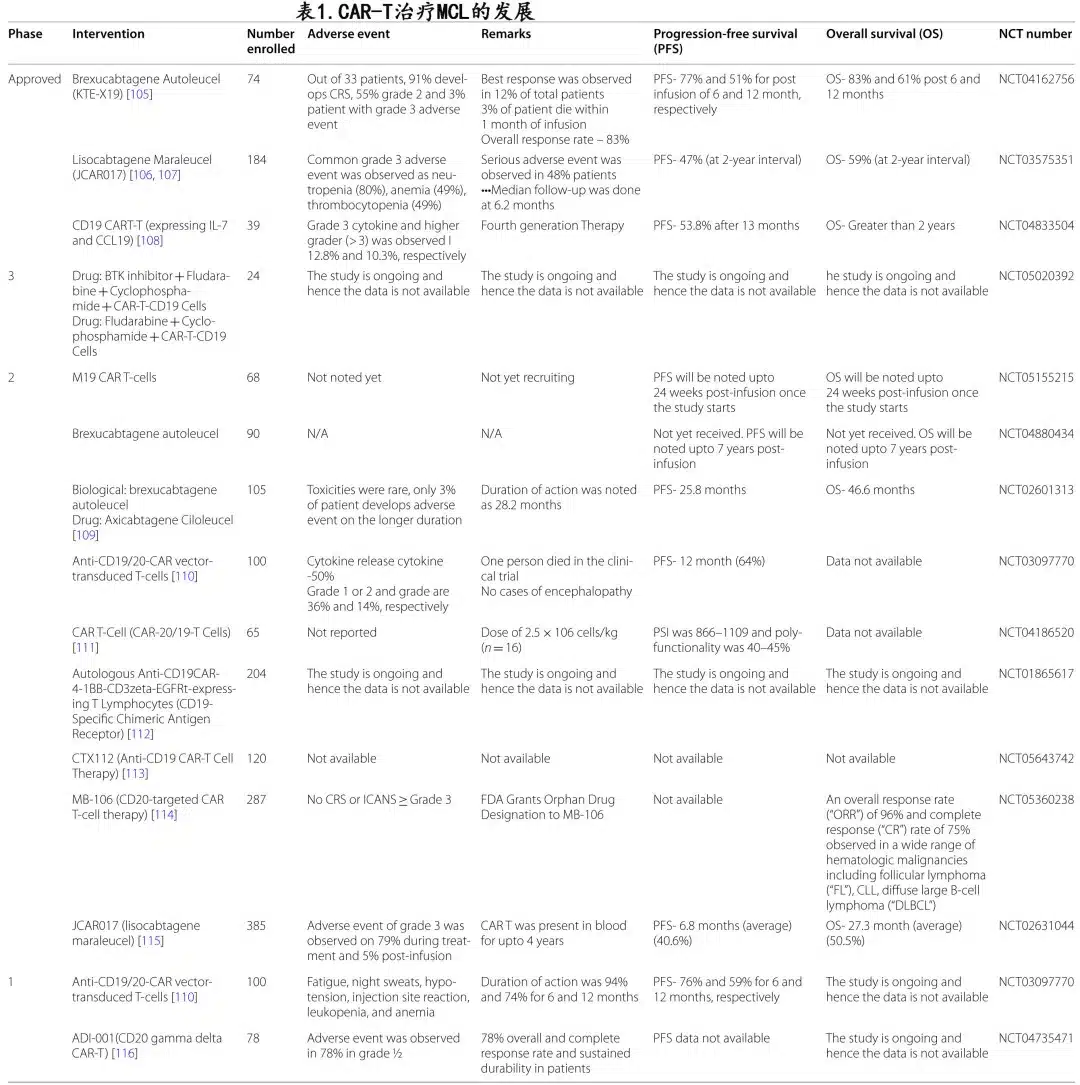
ZUMA-2 is a multicenter phase II study evaluating the efficacy of KTE-X19 (brexucabtagene autoleucel, a CD28 costimulatory domain CD19 CAR-T cell) in R/R MCL patients, with a phase 2 target dose of 2×10^6 CAR+ T cells/kg. Significant response rates were observed, with no statistical significance across any specific adverse prognostic subgroups: brexucabtagene autoleucel was safe and effective in elderly patients, blastoid variant, high Ki-67 proliferative index, high MIPI scores, and TP53 mutant patients, with response rates maintained.
Leading the ZUMA-2 study, Dr. Michael Wang believed CAR-T cells have the ability to control MCL’s resistance compared to other available drugs. ZUMA-2 evaluated KTE-X19 in 60 MCL patients having received up to 5 prior therapies, resulting in an ORR of 93% and CR of 67%. However, in the intention-to-treat evaluation of all 74 patients, the ORR was 85% with 59% achieving CR. Brexu-cel induced rapid responses, with a median time to response of 1 month and median time to CR of 3 months.
At the 47th EHA meeting, investigators reported safety and efficacy results as of December 2019. Based on a median follow-up of 17.5 months, 29 patients (48%) remained in response, including 70% of those achieving CR. With longer follow-up, brexu-cel responses remained consistent. At 32.3 months of follow-up, 39% of the first 28 patients enrolled maintained a response. While the durability of brexu-cel is not fully known, its performance in some high-risk, BTKi-refractory patients has been encouraging thus far.
Dr. Frederick Locke from Moffitt Cancer Center stated that ZUMA-2 achieved significant and durable responses, and when assessing sustained response rates stratified by high-risk or other features typically associated with poor MCL outcomes, there was no statistical significance across any adverse prognostic subgroup: long-term response rates were similar in patients ≥65 years, those with blastoid variant, high Ki-67 proliferative index, TP53 mutation, and high/intermediate MIPI risk scores. KTE-X19 CAR-T cell therapy demonstrated excellent long-term response rates even among high-risk patients. All patients receiving KTE-X19 experienced at least one AE, with 99% having ≥Grade 3 AEs, most typically hematologic toxicities. Two patients (3%) who concurrently received bridging chemotherapy experienced Grade 5 AEs; in one case, sepsis was related to both chemotherapy and brexu-cel infusion.
AEs of special interest with all CAR-T cell therapies include immune effector cell-associated neurotoxicity syndrome and cytokine release syndrome (CRS). The overall CRS rate was 91%, with 15% ≥Grade 3. To treat CRS, 59% of patients received tocilizumab and 22% received glucocorticoids.
As brexu-cel selectively targets CD19+ cells, B-cell aplasia is an expected adverse event that can lead to hypogammaglobulinemia. Flow cytometry analysis showed that patients achieving an objective response and effective CAR-T cell expansion during the initial evaluation period experienced B-cell aplasia. Conversely, non-responders did not develop B-cell aplasia during the study. However, with longer follow-up, B-cell recovery occurred by 12 months in patients with sustained responses.
The TRANSCEND study (lisocabtagene maraleucel (liso-cel)) enrolled MCL patients. As of December 2020, 41 patients completed leukapheresis and 32 received liso-cel infusion. Liso-cel was highly effective in treated patients, with an ORR of 84% and CR of 59%; the blastoid morphologic response rate was 75%. Liso-cel demonstrated excellent safety, with 84% experiencing ≥Grade 3 TEAEs, mostly hematologic abnormalities as ≥Grade 3 events, and 34% having ≥Grade 3 hematologic toxicities persisting beyond Day 29 post-infusion.
Here is my attempt at a precise English translation of the provided text, maintaining all content including references to figures and with accurate translation of medical terminology:
Haploidentical CAR-T cells, derived from partially matched healthy donors such as siblings or children, have been shown to be effective for refractory MCL patients. Haplo-CAR-T cells can proliferate effectively in vivo, exhibiting clinically significant anti-tumor activity without severe adverse effects. In one reported case, the patient achieved partial remission with a minimal residual disease focus.
Limitations of CAR-T Cell Therapy
CAR-T cell therapy is associated with certain adverse effects. Furthermore, despite its efficacy in relapsed or refractory B-cell malignancies, a significant relapse rate exists. Severe adverse effects, limited anti-tumor activity, antigen escape, limited tumor infiltration, and transport limitations are obstacles to the success of CAR-T cell therapy. Many factors can lead to initial CAR-T cell therapy failure. For some patients, post-infusion or manufacturing issues result in inadequate in vivo expansion of CAR-T cells. Other issues include improper CAR-T cell production or underlying patient conditions. An additional factor contributing to initial CAR-T cell therapy failure or limitation is a lack of sufficient research to maximize the therapeutic effect of CAR-T cells.
Antigen escape or tumor resistance to single-antigen targeted CAR constructs is a major limitation of CAR-T cell therapy, and many techniques are now aimed at reducing this limitation by targeting multiple antigens. To simultaneously target multiple tumor antigens, tandem CARs, consisting of a single CAR structure with two scFvs, or dual CAR structures are employed. Clinical studies using CD19 and CD20 or CD19 and CD22 have shown the potential for durable complete remissions. T cell and CAR-T cell metabolic reprogramming can also improve TME responsiveness, activity, and effector functions, or mitigate certain TME-specific changes induced by tumor cells on invading T lymphocytes.
Off-tumor/on-target recognition, insertional oncogenesis, anaphylaxis, graft-versus-host disease, and off-target antigen recognition are some common adverse effects associated with CAR-T cell therapy. Antigen selection in CAR design is crucial to ensure therapeutic efficacy and prevent “on-target off-tumor” toxicity. For MCL, the main targets are CD19 and CD22, which are also expressed on healthy normal B cells. CAR-T cells targeting these antigens will eliminate normal B cells, making B-cell aplasia an indicator of the efficacy, durability, and success rate of CD19 and CD22 CAR-T cell therapies. This limitation can be overcome by designing TME-specific CAR-T cell therapies (e.g., targeting TME hypoxia) aimed at restricting CAR expression to CAR-T cells residing in the hypoxic TME (but not CAR-T cells in non-malignant, normoxic tissues). Targeting tumor-specific post-translational modifications (such as overexpressed truncated O-glycans in solid tumors) may be another approach to circumvent this limitation.
Furthermore, the interactions between the tumor microenvironment, host, and CAR-T cells significantly impact CAR-T cell function. T cell therapy-associated adverse effects can be delayed, acute, severe, mild, or persistent throughout the transgenic T cell lifespan. Toxicities depend on CAR-T cell interactions with patient-specific proteins and fall into two categories: one mediated by CAR-T cell activation and cytokine storm generation. Cytokine release syndrome (CRS), neurotoxicity, macrophage activation syndrome (MAS), and hemophagocytic lymphohistiocytosis (HLH) are manifestations of systemic cytokine toxicity from CAR-T cells. CRS results from in vivo CAR-T cell expansion, triggering the release of multiple cytokines and a systemic inflammatory response, with grading scales including the Lee criteria, American Society for Transplantation and Cellular Therapy (ASTCT), CARTOX (CAR T-cell-therapy-associated TOXicity Working Group), and Penn grading. The ASTCT’s grading of neurotoxicity is termed immune effector cell-associated neurotoxicity syndrome (ICANS), with symptoms ranging from fatigue, psychosis, encephalopathy, ataxia, seizures, agitation, and (rarely) cerebral edema. ICANS is graded using the 10-point Immune Effector Cell-Associated Encephalopathy (ICE) scale.
Fever, hypotension, infection, encephalopathy, fatigue, tachycardia, and arrhythmia are typical adverse effects of brexucabtagene, with most occurring within the first two weeks of treatment but some potentially arising later.
In Professor Michael Wang’s study, 3 out of 60 patients died from treatment-related adverse effects. Additionally, patients denied CAR-T cell therapy may die from MCL in less than a year. Further research is needed to confirm the average duration of treatment required for patients. This ability of CAR-T cells seems to be another application of personalized immunotherapy against cancer, while also leveraging this promising new scientific breakthrough in medicine. KTE-X19 carries the risk of cytokine release syndrome (a potentially fatal condition) and neurological adverse reactions and is only available through a Risk Evaluation and Mitigation Strategy (REMS) program.
Zanubrutinib, Ibrutinib, Acalabrutinib, and Orelabrutinib have changed the treatment landscape for R/R MCL, and CAR-T cells are crucial for patients refractory to BTK inhibitors, who have a poorer overall prognosis, with some rapidly progressing to more aggressive disease forms after BTK inhibitor therapy. CAR-T represents a significant advancement in the treatment of relapsed and refractory MCL and should be evaluated in all patients.
Management of Toxicities and Resistance in CAR-T Cell Therapy
While CAR-T cell therapy has achieved the above successes, there remains a high likelihood of inducing various adverse effects, such as neurotoxicity, cytokine release syndrome, on-target/off-tumor recognition, insertional oncogenesis, graft-versus-host disease, and acute anaphylaxis. Like axicabtagene ciloleucel, brexucabtagene autoleucel has certain adverse effects, including cytokine release syndrome and neuronal toxicity, but these adverse effects are manageable and self-limiting. To overcome systemic cytokine toxicity, researchers have introduced different approaches, such as CAR subdimer dimerization switches, CAR-T cells with small molecule adapter agents for off/on gating of functions, CAR downstream signaling inhibitors, the use of protease inhibitors to control CAR protein expression, and engineered CAR-T cell modifications with synthetic factors (to counter cytokine storm and control CAR expression).
Anti-Thymocyte Globulin (ATG) for Managing Neurotoxicity
Cerebral edema in R/R MCL patients receiving CAR-T cell therapy can be potentially fatal, and one study suggests that CAR-T cell therapy-induced cerebral edema can be fully reversed through multi-modal clinical interventions, including anti-thymocyte globulin (ATG). Following ATG administration, biomarker data showed an initial and robust CAR-T cell expansion and associated inflammatory cytokine generation, followed by a rapid decline in CAR-T cells and pro-inflammatory cytokine levels. This clinical data provides promising information on the potential of ATG in managing severe CAR-T cell-induced neurotoxicity.
Engineering CAR-T Cells to Manage Toxicity
To achieve therapeutic benefit, CAR-T cells must remain within their therapeutic window, as exceeding this window can lead to toxicity. The amount of tumor antigens produced by cancer cells, tumor burden, the affinity of the antigen-binding domain with its epitope, and the co-stimulatory components of the CAR all influence the activation rate of CAR-T cells. To maximize therapeutic efficacy and minimize toxicity, a careful analysis of the many modular components of the CAR is crucial. It is expected that as the affinity of the antigen-binding domain decreases, a higher antigen density on tumor cells is required to achieve significant activation levels. Antigen-binding structural domains with micromolar affinities have higher selectivity for cancers expressing higher levels of the target antigen compared to those with low nanomolar/subnanomolar binding affinities. The production of cytokines can also be controlled by modifying the hinge and transmembrane regions that activate CAR-T cells. Changes in the CD8α-derived transmembrane amino acid sequence and hinge regions of the CD19-targeted CAR can reduce cytokine release and CAR-T cell proliferation. Another customizable domain in CAR design is the co-stimulatory domain, such as using less differentiated T cell subsets or designing CAR-T cells with 4-1BB ligands to create a supportive environment for CAR-T cells, which can improve their in vivo efficacy. Overcoming the physical barriers and antigen heterogeneity of solid tumors can also improve CAR-T cell infiltration into solid tumors.
Managing CAR Immunogenicity
The general toxicity of traditional chemotherapeutic drugs can be lowered by modifying immune cells to recognize their targets, while drug immunosuppression, targeted activation, and the expression of some elimination genes are common practices to minimize adverse reactions to CAR-T cell therapy. Using human or humanized antibody fragments instead of murine-derived CARs may help lower CAR immunogenicity, as the host immune system’s detection of CAR structures can promote cytokine-related toxicity.
Modifying CAR-Transduced T Cells to Manage Neurotoxicity
Myeloid cells and cytokines appear to play a critical role in CAR-T cell-induced neurotoxicity, as CD14+ cells are significantly increased in patients with ≥ grade 3 neurotoxicity. According to a pivotal CAR-T cell study in large B-cell lymphoma, elevated GM-CSF levels were closely associated with the occurrence of ≥ grade 3 neurotoxicity. In preclinical studies, Lenzilumab can inhibit the granulocyte-macrophage colony-stimulating factor (GM-CSF), thereby reducing neurotoxicity and CRS while increasing CAR-T cell activation. In CAR-transduced T cells, GM-CSF inactivating mutations appear to produce similar results, suggesting that GM-CSF neutralization helps reduce neurotoxicity and CRS. Additionally, when tyrosine hydroxylase is deficient in a bone marrow cell-specific manner or the enzyme is inhibited by tyrosine inhibitors, catecholamine and cytokine levels decrease. Furthermore, according to preclinical studies, neuroinflammation in CD19 CAR treatment of leukemia/lymphoma animal models is reduced when an IL-1 receptor antagonist is used.
Suicide Gene Strategies for CARs
Suicide genes or “off-switch” technologies can more easily selectively reduce engineered cells when adverse events start to occur by using secondary inducers. For example, designing CARs to express full-length CD20 or a CD20 mimetic epitope can help eliminate CAR-T cells using Rituximab. The main disadvantage of these therapies or their equivalent treatments is that, while they are necessary to ensure safety, their use abruptly ends the treatment (of a rapidly progressing disease). Regardless, this limitation provides a strong incentive for developing enhanced safety technologies (while retaining suicide gene activation as a last resort).
To enhance outcomes and move beyond the limitations of CAR-T cells, proactive and appropriate efforts must be made from patient identification to isolation, production, expansion, and infusion of CAR-T cells. To avoid limitations or failure of CAR-T cell therapy, it is crucial to monitor CAR-T cell efficacy and antigen loss throughout the treatment period. Improvements to traditional CAR-T cell designs aim to overcome the shortcomings or adverse reactions of this therapy. To evaluate the long-term protective effect of KTE-X19, the FDA mandated that the manufacturing company initiate a drug vigilance study for patients receiving KTE-X19 treatment. Due to the high cost and labor requirements, research has begun to focus on allogeneic CAR-T infusions, which are currently in different stages of clinical development. The infusion of CAR-T cells is typically the bottleneck that hinders further progress and leads to cell toxicity. However, CAR-T cell therapy is still relatively new, and the widespread application of CAR-T cell therapy will undoubtedly face numerous scientific knowledge challenges before it can be widely used.
Summary
MCL is a pathologically distinct, life-threatening disease that requires innovative treatments, and fortunately, the development of CAR-T cell approaches and cellular immunotherapies has opened a new world of treatment options. Researchers are investigating CAR-T cell therapies for different types of tumors, but the most successful CAR-T cell therapies to date have been for hematological malignancies. Most CAR-T cell therapies carry the potential for severe side effects that must be handled quickly and cautiously by medical professionals skilled in the procedure. Additionally, patients and healthcare providers face different logistical and financial challenges. Severe toxicities caused by CAR-T cell therapy, such as cytokine storms, macrophage activation syndrome, neurotoxicity, and HLH, are also unavoidable obstacles. To minimize these events, researchers are striving to improve the safety of CAR-T cell therapy through safer antigen selection or by modifying the sensitivity of the antibody single-chain variable fragment, or by controlling CAR-T cell activity through combined antigen targets and CAR masking, or by introducing suicide genes and limiting CAR expression.
Brexu-cel treatment for R/R MCL in the real world in the United States resulted in an ORR of 84% for all patients and 78% for patients with follow-up less than 180 days. The 6-month OS and PFS rates were 79% and 66%, respectively. After receiving Tocilizumab and Corticosteroids, ≥ grade 3 CRS (9%) and ICANS (29%) mostly resolved within 21 days of treatment initiation. 32% of patients experienced acquired severe infections, 22% experienced chronic cytopenias, and 3% subsequently developed malignancies. This is the most comprehensive report on the application of brexu-cel, indicating successful CAR-T cell therapy for MCL (including MCL with high-risk features), but further research is needed on severe toxicities.
Although further research on MCL treatment is still needed, these trials hold promise for bringing new drugs for lymphoma treatment and providing better cures for patients with advanced tumors. These studies will pave the way for research into different types of lymphomas, changing the lives of these previously difficult-to-treat patients.
References
Zoufang Huang,et al. CAR T-Cell therapy for the management of mantle cell lymphoma.Mol Cancer . 2023 Mar 31;22(1):67. doi: 10.1186/s12943-023-01755-5.
Content Source:聊聊血液
