CAR-T NHL, The International First Double Negative CAR-T Cell Therapy for Lymphoma Research Results Released, Safe and Effective
CAR-T NHL, The International First Double Negative CAR-T Cell Therapy for Lymphoma Research Results Released, Safe and Effective
CAR-T cell therapy is an innovative treatment for cancer. The million-dollar CAR-T cell injection has saved the lives of countless patients with difficult-to-treat, relapsed lymphomas, including diffuse large B-cell lymphoma (DLBCL). However, autologous CD19 CAR-T cell therapy is a personalized cellular treatment with high costs and limited clinical accessibility that needs improvement.
On February 29, 2024, the Cellular Therapy Team of the Department of Hematology at the Second Affiliated Hospital of Zhejiang University published a research paper titled “CD19-CAR-DNT cells (RJMty19) in patients with relapsed or refractory large B-cell lymphoma: a phase 1, first-in-human study” in the top-tier medical journal eClinical Medicine (IF=15.1), a Lancet subsidiary. This paper is the first international report of the clinical trial results for dual-negative CAR-T cells (CD19-CAR-DNT), a universal CAR-T cell product. The study demonstrated the good tolerability, safety, and preliminary efficacy of CD19-CAR-DNT cell infusion in non-Hodgkin’s lymphoma.

Diffuse large B-cell lymphoma (DLBCL) is the most common clinically aggressive B-cell malignancy. Patients with refractory or relapsed DLBCL have a poor prognosis, seriously threatening their health. In recent years, chimeric antigen receptor (CAR-T) therapy targeting CD19 has achieved tremendous success in treating DLBCL, with long-term survival exceeding 40% for autologous CD19-specific CAR-T cell therapy in difficult-to-treat, relapsed large B-cell lymphoma (LBCL). However, the high cost of personalized autologous CAR-T cell therapy, treatment failure due to impaired patient T-cell function, and toxic side effects such as cytokine release syndrome (CRS) have limited its widespread clinical application. To overcome the limitations of autologous CAR-T cell therapy, allogeneic universal CAR immune cells may represent a potential new cellular therapy option for treating cancer.
Compared to autologous CAR-T cells, allogeneic immune cells offer several advantages:
(1) Healthier donor cells can provide more functional immune cell products, avoiding T-cell dysfunction;
(2) The risk of manufacturing failure can be avoided;
(3) They possess the characteristics of an off-the-shelf, universal product, which can shorten the time to patient treatment.
A subset of T cells in human peripheral blood, called double-negative T cells (DNTs), does not express CD4 and CD8 molecules. DNTs can overcome graft-versus-host disease (GVHD) and host-versus-graft (HVG) reactions, making them candidates for “universal” CAR immune cell therapy. The off-the-shelf, universal allogeneic CD19-CAR-DNT cell product (RJMty19 injection) is a new immune cell therapy designed to treat patients with relapsed/refractory large B-cell lymphoma (LBCL), addressing the pain points and challenges of autologous CAR-T cell products and meeting the current clinical needs in the field of hematological malignancies.
Preclinical studies have shown that CD19-CAR-DNT cells can effectively kill CD19+ cell lines (NAML-6 and Daudi) in vitro and in vivo, significantly prolonging the survival of NAML-6 tumor-bearing mice. Compared to conventional CAR-T cells, CD19-CAR-DNT cells demonstrated similar tumor-killing effects against NAML-6 and Daudi cell lines. Therefore, the Department of Hematology at the Second Affiliated Hospital of Zhejiang University conducted an investigator-initiated, dose-escalation phase 1 clinical trial (clinical trial registration number: NCT05453669) using allogeneic CD19-CAR-DNT cells for the treatment of large B-cell lymphoma.
The study design included five dose cohorts (1×10^6, 3×10^6, 9×10^6, 2×10^7, and 3×10^7 CAR+ cells/kg). Dose-limiting toxicity (DLT) evaluation has been completed for the first 13 patients in the five dose cohorts of 1×10^6, 3×10^6, 9×10^6, 2×10^7, and 3×10^7 CAR+ cells/kg. No ≥Grade 3 CRS, ICANS, GVHD, or serious adverse events (SAEs) were observed, indicating good clinical safety. In terms of clinical efficacy, as the dose increased, the clinical response improved significantly. The clinical study found that in the fourth dose cohort (2×10^7 CAR+ cells/kg), all three subjects achieved a response after a single infusion, with an overall response rate (ORR) of 100% (3/3) and a complete response rate (CRR) of 33.3% (1/3). These data suggest that CD19-CAR-DNT cell therapy for LBCL has good safety and higher doses are effective.
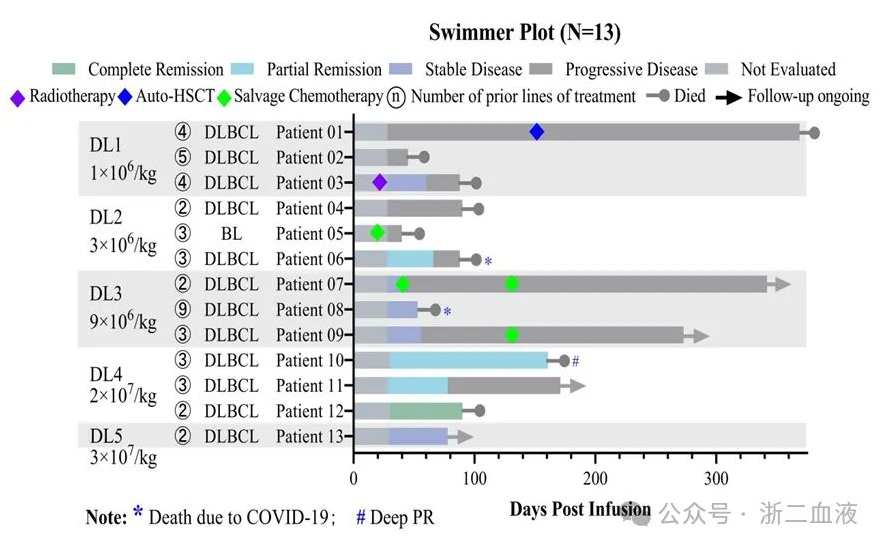
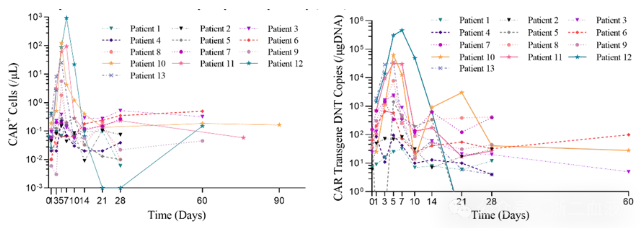
Pharmacokinetic studies using flow cytometry and quantitative PCR demonstrated that CD19-CAR-DNT cells expanded in a dose-dependent manner in vivo, reaching peak levels 5-7 days after infusion. One subject achieved a maximum peak level of 936.4 CAR+ cells/μl whole blood, and the average peak level in the fourth dose cohort (2×10^7 CAR+ cells/kg) was 384.2 CAR+ cells/μl whole blood. This indicates that higher doses of CD19-CAR-DNT cells achieved better in vivo expansion, with the average peak level in the 2×10^7 CAR+ cells/kg dose cohort (384.2 CAR+ cells/μl whole blood) exceeding the average peak level of the approved CD19-CAR-T cell product (43.6 CAR+ cells/μl whole blood for patients who achieved a response). These results suggest that high-dose CD19-CAR-DNT cell infusion can achieve relatively good in vivo expansion.
This trial demonstrated that CD19-CAR-DNT cells have good tolerability and safety, and have shown promising anti-tumor activity in LBCL patients. Unfortunately, our study also indicated that the durability of the clinical efficacy of CAR-DNT cells needs further improvement. We look forward to the future phase 2 expansion clinical study with an optimized multiple-dosing regimen of CD19-CAR-DNT cells, which may provide a new cellular therapy option and benefit patients with difficult-to-treat or relapsed large B-cell lymphoma.
Professor Qian Wenbin from the Department of Hematology at the Second Affiliated Hospital of Zhejiang University Medical College is the corresponding author of this paper. Dr. Xiao Xibin, Associate Chief Physician, and Dr. Liu Hui, Ph.D., from the Department of Hematology at the Second Affiliated Hospital of Zhejiang University Medical College, are co-first authors.
Content Source:浙二血液
