FDA Yescarta (Axicabtagene Ciloleucel, Axi-Cel) Approval for Large B-Cell Lymphoma on April 1, 2022
FDA Yescarta (Axicabtagene Ciloleucel, Axi-Cel) Approval for Large B-Cell Lymphoma on April 1, 2022
On April 1, 2022, the FDA announced the approval of a new second-line treatment indication for Axicabtagene Ciloleucel (Yescarta, Axi-Cel) for adult patients with large B-cell lymphoma that is refractory to or relapses within 12 months after first-line chemoimmunotherapy. It’s important to note that this therapy is not suitable for patients with primary central nervous system lymphoma.
This approval was based on the results of the ZUMA-7 trial. The enrolled patients had not yet received treatment for relapsed or refractory disease and were potential candidates for autologous hematopoietic stem cell transplantation (HSCT).
A total of 359 patients participated in the clinical trial. They were divided into a trial group and a control group. The trial group received a single infusion of Yescarta after fludarabine and cyclophosphamide (Flu/Cy) lymphodepleting chemotherapy, while the control group received 2 or 3 cycles of standard chemotherapy followed by high-dose therapy and autologous stem cell transplantation, based on the physician’s assessment of their condition.
The results showed that patients receiving Yescarta treatment had an 18-month event-free survival rate of 41.5%, while the standard treatment control group had only 17.0%. The median event-free survival for Yescarta-treated patients was 8.3 months, compared to 2.0 months for the control group.
In terms of response rates, the overall response rate for Yescarta-treated patients was 83%, including 65% complete responses and 18% partial responses. The control group had an overall response rate of only 50%, including 32% complete responses and 18% partial responses.
Regarding survival, the median progression-free survival for Yescarta-treated patients was 14.7 months, and the median overall survival exceeded 36 months, with more than 50% of patients still alive. In contrast, the control group had a median progression-free survival of 3.7 months and a median overall survival of 35.1 months.
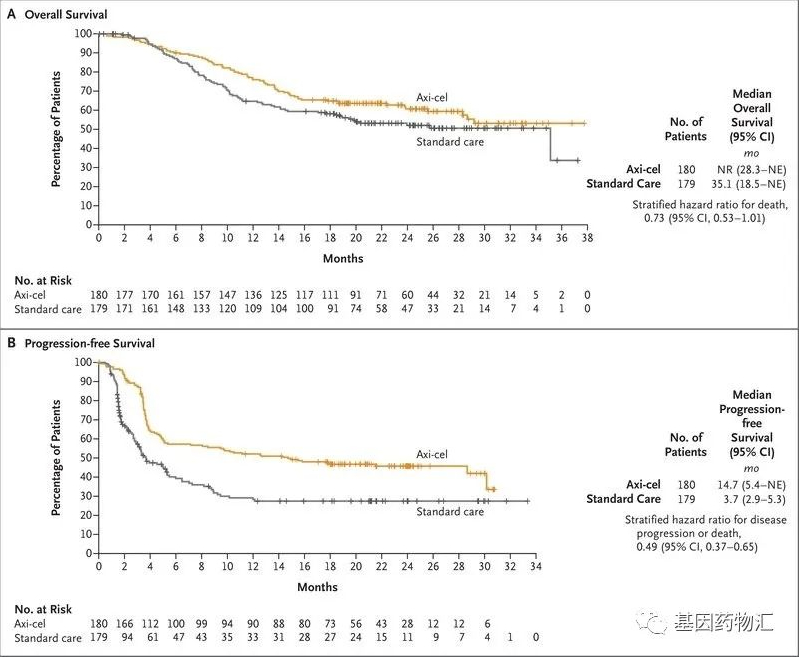
Image from a published paper
In terms of adverse events, the incidence of grade 3 or higher severe adverse events was 91% in Yescarta-treated patients and 83% in the control group.
Overall, compared to the existing standard of care, including autologous stem cell transplantation, Yescarta significantly improved event-free survival and enabled more patients to benefit from the treatment.
