T Cell Lymphoma CAR-T Therapy Achieves Complete Remission in 28 Days
T Cell Lymphoma CAR-T Therapy Achieves Complete Remission in 28 Days
In early June 2024, the results of a Phase I clinical trial for the CD7-CAR-T product PA3-17 in the treatment of relapsed/refractory T-cell acute lymphoblastic leukemia/lymphoblastic lymphoma (r/r T-ALL/LBL) were presented at the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting.

Source: ASCO screenshot, contact for removal if copyrighted
PA3-17 injection is the world’s first autologous CD7-CAR-T product to receive IND approval. In November 2021 and December 2022, PA3-17 injection was granted Orphan Drug Designation by the U.S. Food and Drug Administration (FDA) and the European Commission (EC), respectively. In August 2021, it received approval from the Center for Drug Evaluation (CDE) of the National Medical Products Administration to enter a registration clinical trial for the treatment of refractory/relapsed T-cell acute lymphoblastic leukemia/lymphoblastic lymphoma. This Phase I trial enrolled a total of 12 patients, with results showing an objective response rate (ORR) of 83.3%, meaning 10 patients achieved remission, of which 9 (75%) achieved complete remission (CR). Among the patients who achieved complete remission, 5 maintained complete remission for over 6 months! Most notably, one patient had a tumor mass larger than 7cm in diameter prior to CAR-T cell therapy, and achieved complete remission 28 days after treatment with PA3-17 injection, indicating that the tumor had completely disappeared!
Ongoing Clinical Trial
Fortunately, the clinical trial of PA3-17 injection is currently recruiting adult patients with relapsed/refractory CD7-positive hematological malignancies. Patients can participate in the clinical trial to receive the drug treatment free of charge after their medical records are reviewed and approved for enrollment. Patients interested in or in need of the clinical trial can click the online consultation link.
Study Title: An Open-Label, Dose-Escalation Phase I Clinical Study of PA3-17 Injection in the Treatment of Adult Patients with Relapsed/Refractory CD7-Positive Hematological Malignancies
Partial Inclusion Criteria:
1. Age 18-70 years;
2. ECOG score 0-1;
3. Confirmed diagnosis of malignant lymphoma, including acute T-cell lymphoblastic leukemia/lymphoblastic lymphoma.
For patients who are not responsive or have poor responses to conventional therapies, clinical trials may offer new hope and treatment opportunities, as well as significantly reduce economic burden for families. Patients interested in or in need of clinical trials can submit their medical records to the Medical Oncology Department (400-880-3716) to find suitable clinical studies.
CAR-T therapy is a “hot” treatment approach that involves extracting a patient’s own T cells, engineering them, and then reinfusing the engineered T cells back into the patient’s body to eliminate cancer cells.
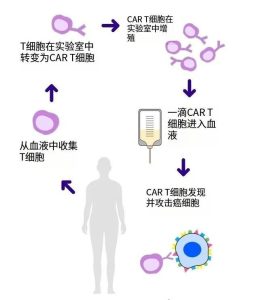
CAR-T Therapy CTX130 Achieves Complete Remission in T-Cell Lymphoma Patient
In the COBALT-LYM trial (NCT04502446), the CAR-T cell therapy CTX130 was studied in patients with T-cell lymphoma (TCL). Preliminary data showed an objective response rate of 70% and a complete remission rate of 30%.

Source: Reference 2 screenshot, contact for removal if copyrighted
In the trial, a female patient with adult T-cell leukemia/lymphoma (ATLL) achieved partial remission 28 days after the first infusion of CAR-T cell therapy CTX130.
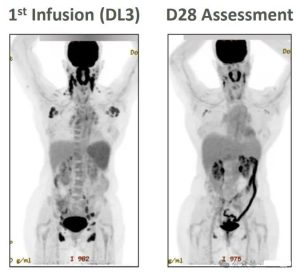
Source: Reference 2 screenshot, contact for removal if copyrighted
After the second infusion of CAR-T cell therapy CTX130, the patient achieved complete remission 28 days later.
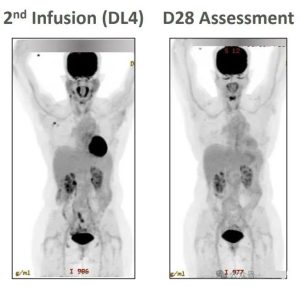
Source: Reference 2 screenshot, contact for removal if copyrighted
This trial demonstrated that CAR-T cell therapy CTX130 can effectively induce remission in T-cell lymphoma.
Conclusion
To date, CAR-T cell therapy has achieved breakthrough success in hematological malignancies, with several approved products for the treatment of multiple myeloma, lymphoblastic leukemia, and others. Although no CAR-T products have been approved for solid tumors yet, new strategies and potential solutions are continuously being developed, which may pave the way for more effective and safer future treatments. CAR-T therapy is being clinically investigated in various solid tumor types, including breast cancer, ovarian cancer, gastric cancer, and prostate cancer, with promising efficacy results. It is believed that in the near future, CAR-T cell therapy will make significant strides in the field of solid tumors, bringing hope to patients with these malignancies!
Reference Material
1.https://ascopubs.org/doi/10.1200/JCO.2024.42.16_suppl.6515
2.THE COBALT-LYM STUDY OF CTX130: A PHASE 1 DOSE ESCALATION STUDY OF CD70-TARGETED ALLOGENEIC CRISPR-CAS9–ENGINEERED CAR T CELLS IN PATIENTS WITH RELAPSED/REFRACTORY (R/R) T-CELL MALIGNANCIES
