China’s new drug Aumolertinib Mesilate Tablets: The world’s second EGFR-targeted therapy for lung cancer with gene mutations.
lung cancer
Aumolertinib Mesilate Tablets (Ameile) were approved for marketing in China on March 18, 2020. It is China’s first third-generation EGFR-targeted therapy for lung cancer with gene mutations and the second such drug globally, following Osimertinib.
On August 20, 2024, the new indication marketing application for Aumolertinib Mesilate Tablets was accepted for priority review by the China Center for Drug Evaluation (CDE). This indication is for the treatment of unresectable locally advanced non-small cell lung cancer (NSCLC) that has not progressed following platinum-based chemoradiotherapy. This is the fourth indication for which the drug has submitted a marketing application, and it is expected to be approved in the second quarter of 2025.
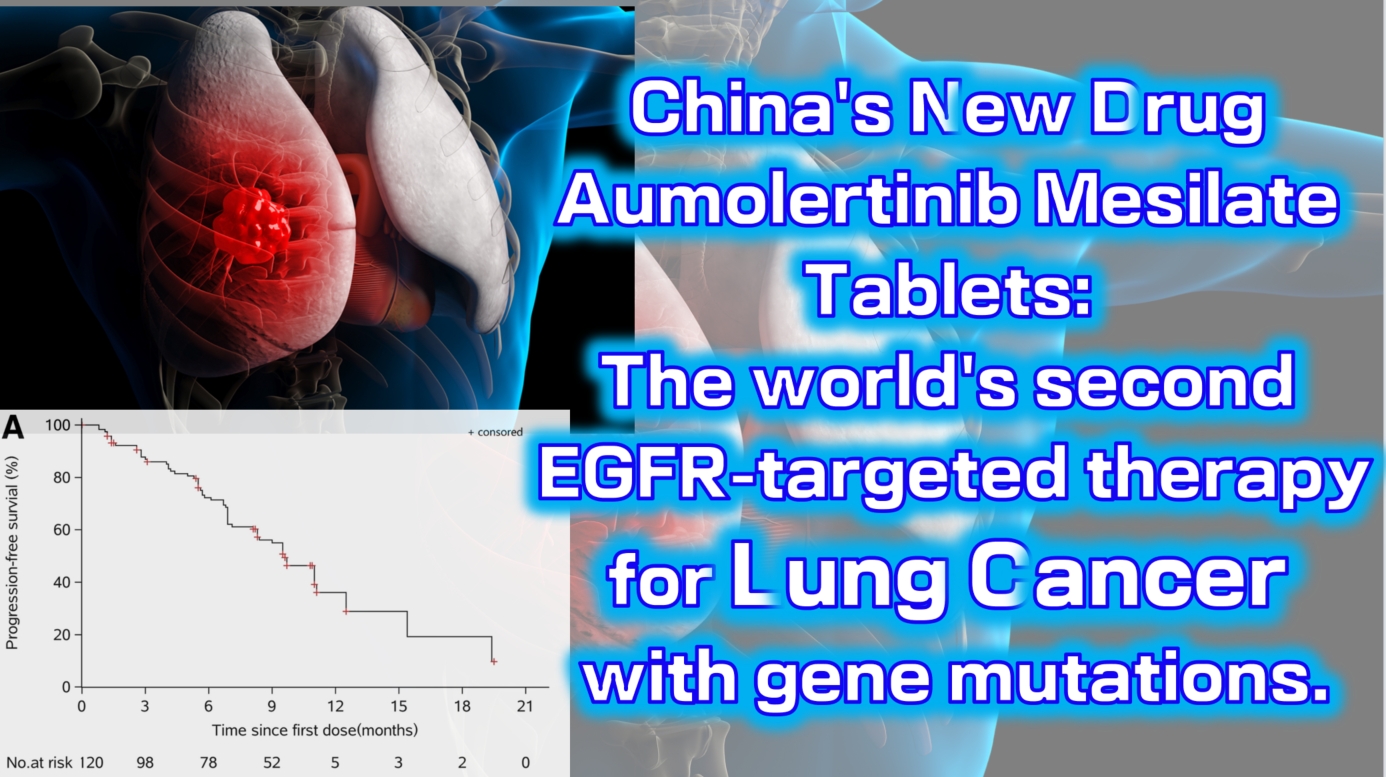
The Phase 1 clinical trial (NCT0298110) enrolled a total of 120 patients with locally advanced or metastatic non-small cell lung cancer (NSCLC). The results showed an objective response rate (ORR) of 52% (range: 42–63), with a disease control rate (DCR) as high as 92% (range: 84–96). The median progression-free survival was 11.0 months (95% CI: 9.5-not reached).

 To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
To assess whether the condition is suitable for CAR-T or clinic therapy, you can submit Advanced Medicine in China for preliminary evaluation!
Email: doctor.huang@globecancer.com

