4 months ago
4 months ago
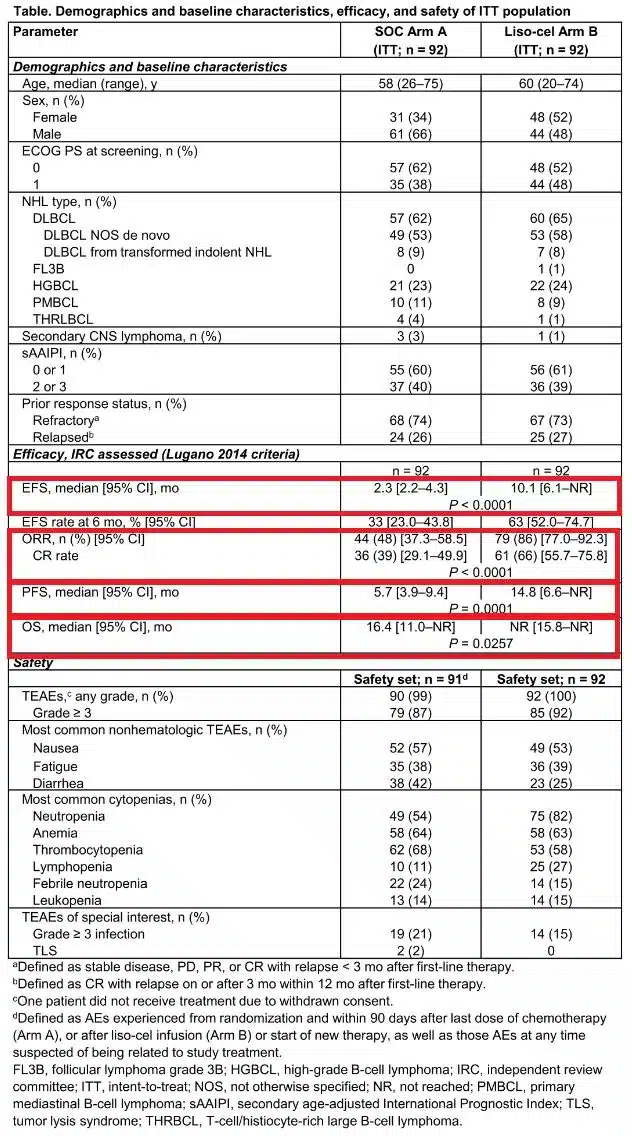
On May 30, 2024, FDA Approves Bristol Myers Squibb’s Breyanzi for the Treatment of Relapsed or Refractory Mantle Cell Lymphoma (MCL)
On May 30, 2024, FDA Approves Bristol Myers Squibb’s Breyanzi for the Treatment of Relapsed or Refractory Mantle Cell Lymphoma (MCL) On May 30, 2024, Bristol Myers Squibb announced that the U...