1 year ago
1 year ago
Equecabtagene Autoleucel (CT103A): A Promising BCMA-Targeted CAR-T Therapy for Multiple Myeloma
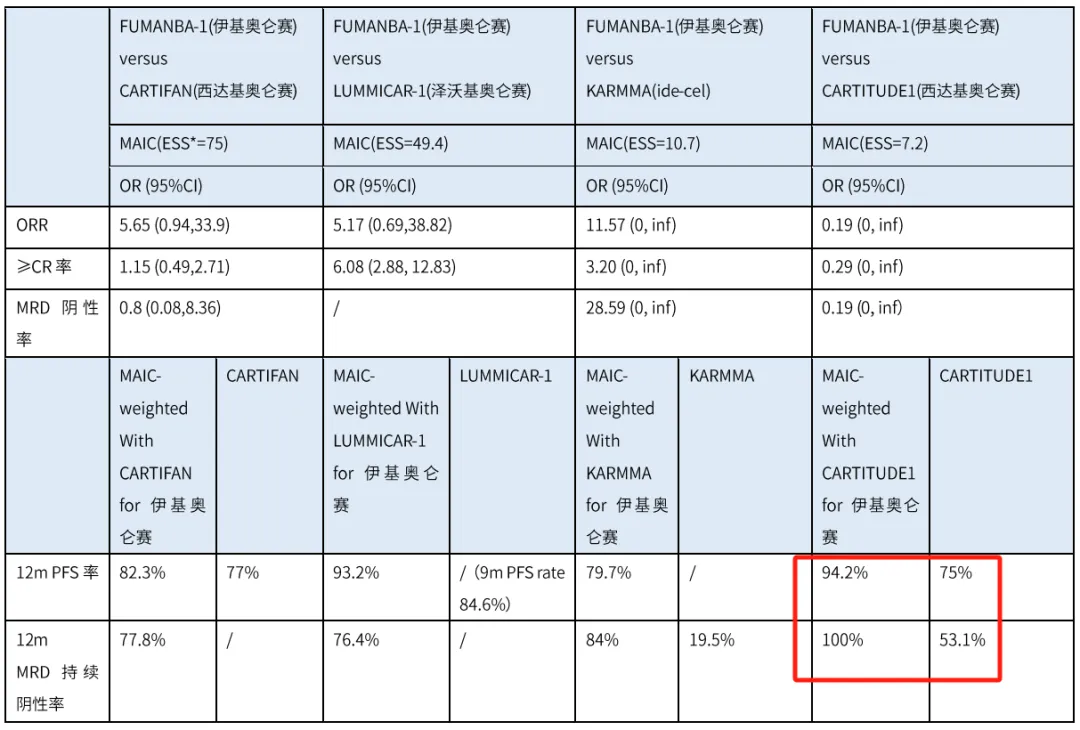
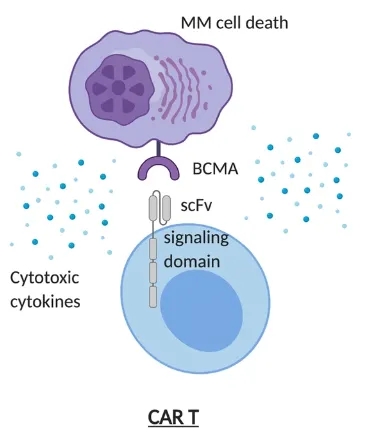
Equecabtagene Autoleucel (CT103A): A Promising BCMA-Targeted CAR-T Therapy for Multiple Myeloma The development of malignant blood disorders, such as multiple myeloma, is associated with immune def...