CAR T-Cell Therapy In China – Recent Advances
August 2023: Cancer patients who previously had few options now have hope because to CAR-T cell therapy, which has revolutionized the area of cancer treatment. With several clinical trials being conducted to increase the efficacy and safety of CAR-T cell therapy, China has emerged as a leader in this ground-breaking sector. These studies demonstrate the nation’s dedication to improving medical research and offering patients cutting-edge care. Currently there are more than 250 ongoing clinical trials on CAR T Cell therapy in China.

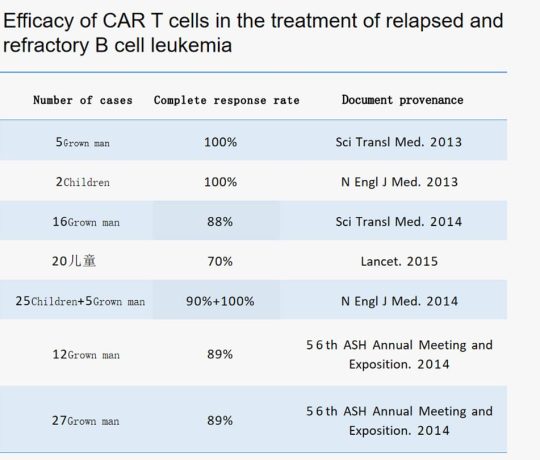

As of January 30, 2018, China registered 171 CAR-T clinical research projects on ClinicalTrial.gov, which has surpassed Europe in number and is second only to the United States, and shows an increasing trend year by year.
What is the scope of CAR-T Cell Therapy in China?
The use of chimeric antigen receptor (CAR) T-cell treatments in the treatment of hematological malignancies has been incredibly effective. The application of CAR T-cell treatments has grown in China over the past nine years.
The first CAR T cell clinical studies started in 2013, and by 2017 there were more CAR T cell clinical trials than ever before. Soon after, China announced that it would provide a total of US$237 billion in funding for cell therapy businesses in 2021, which represented a huge increase in the number of clinical trials and fundamental research projects involving CAR T cells.
Strong government support, money inflow, high patient demand, a distinctive healthcare system, and the efforts of Chinese doctors and scientists all contributed to this significant surge in activity in China.
Why choose China for CAR T-Cell therapy?

Clinical trials and advance research
With more 350 ongoing clinical trials on CAR T-Cell therapy, China has achieved great strides. Innovative CAR T-cell therapies have been actively developed and tested by Chinese researchers and medical institutes, with a focus on both solid tumours and haematological malignancies. In terms of CAR T cell therapy developments, China has been in the vanguard, and there are numerous cutting-edge clinical trials happening there. Because of this, patients looking to access the most recent developments in CAR T cell therapy may find China to be an appealing alternative..
Cost and availability
CAR T-Cell therapy cost in China is significantly lower than that in countries like US, UK, Australia, Japan, Korea and Singapore. CAR T-Cell therapy in China may cost just $ 60,000 USD. China has a comparatively high availability of CAR T cell therapy. There may be shorter treatment wait periods now that more medical facilities in China have received regulatory authorization to provide CAR T cell therapy. In addition, CAR T cell therapy is frequently less expensive in China than it is in other places, like the United States. This element may be especially important for people looking for affordable treatment choices.


Expertise and experience
Due to its participation in multiple clinical trials and therapies, China has developed a significant amount of knowledge and experience in providing CAR T cell therapy. Chinese medical experts have improved their knowledge of CAR T cell therapy-related problems and their ability to manage them in order to improve patient outcomes. China offers high-quality healthcare thanks to its vast patient population, supportive infrastructure, and collected knowledge in CAR T cell therapy.
Companies in US collaborating with Chinese companies
In recent years, American businesses have begun collaborating with their Chinese counterparts to develop CAR T cell medicines. For ex. In 2015, WuXi AppTec, a Chinese contract research organisation (CRO), and Juno Therapeutics, a US-based biopharmaceutical business, partnered strategically. Chinese biopharmaceutical company Cellular Biomedicine Group worked with US biopharmaceutical company Celgene, which is now a division of Bristol-Myers Squibb. A global partnership and licence deal was made between Janssen Biotech, a pharmaceutical firm owned by Johnson & Johnson, and Legend Biotech, a business with operations in both China and the US.

CAR-T products have been marketed worldwide
| Listed country | Drug name | Price | Target point | Time to market | Research and development company | indication |
|---|---|---|---|---|---|---|
| China | Inaticabtagene Autoleucel(CNCT19) | $136,111 | CD19 | 2023.11 | Juventas | the treatment of adult B-cell relapsed and refractory acute lymphoblastic leukemia (B-ALL). |
| Equecabtagene Autoleucel(FUCASO) | $160,308 | BCMA | 2023.7 | IASO BioTherapeutics | relapsed or refractory multiple myeloma who previously received 3 or more lines of therapy. | |
| Axicabtagene ciloleucel(yescarta) | $164,983 | CD19 | 2021.6 | Fosun Kite | adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy | |
| Relmacabtagene Autoleucel(Carteyva) | $177,356 | CD19 | 2022.10 | JW Therapeutics | the treatment of adult patients with follicular lymphoma that is refractory or that relapses within 24 months of second-line or above systemic treatment (r/r FL). | |
| America | Abecma | $438,000 | BCMA | 2021.3 | BristolMyers Squibb | Treat adult patients with multiple myeloma who have not responded to, or whose disease has returned after, at least four prior lines (different types) of therapy. |
| Carvykti | $465,000 | BCMA | 2022.2 | Legend Biotech | the treatment of adult patients with relapsed or refractory multiple myeloma after four or more prior lines of therapy. | |
| Breyanzi | $410,300 | CD19 | 2021.2 | BristolMyers Squibb | to treat adult patients with certain types of large B-cell lymphoma | |
| Tecartus | $373,000 | CD19 | 2020.7 | Gilead Sciences | Relapsed or Refractory Mantle Cell Lymphoma | |
| Yescarta | $373,000 | CD19 | 2017.10 | Kite Pharma | to treat adult patients with certain types of large B-cell lymphoma who have not responded to or who have relapsed after at least two other kinds of treatment. | |
| Kymriah | $475,000 | CD19 | 2017.8 | Novartis | for certain pediatric and young adult patients with a form of acute lymphoblastic leukemia (ALL). |
-
The US FDA approved the market of CAR-T
-
CAR-T approved for market in China
 1Whole-process management
1Whole-process management 1.Disease analysis
2. Create a file
3. Sorting out medical records
4. Preliminary assessment
5. Consult and arrange living assistance
6. Disease follow-up
7. Get insurance 2Expert consultation assessment
2Expert consultation assessment
1.Medical record collection
2.Translation of medical records
3.Text consultation
4.Video consultation 3Ground access service
3Ground access service
1. Apply for hospital invitation letter
2. Apply for a visa
3. Book a hotel
4. Airport pick-up and transportation
5. Mobile card processing
6. Daily living assistance
7. Daily travel booking and assistance 4Medical service
4Medical service
1. Designated hospitals are green
2. Assist in arranging examinations, hospitalization and treatment
3. Interpreter accompanied by hospital
4. Assist in issuing bills 5CAR-T therapy
5CAR-T therapy
making a treatment plan1.CART cell technology Services
 6Family accommodation
6Family accommodation
1. Based on 2 persons/month
2. Provide accommodation assistance to overseas personnel
 1Whole-process management
1Whole-process management
1.Disease analysis 2. Create a file 3. Sorting out medical records 4. Preliminary assessment 5. Consult and arrange living assistance 6. Disease follow-up 7. Get insurance 2Expert consultation assessment
2Expert consultation assessment
1.Medical record collection 2.Translation of medical records 3.Text consultation 4.Video consultation 3Ground access service
3Ground access service
1. Apply for hospital invitation letter 2. Apply for a visa 3. Book a hotel 4. Airport pick-up and transportation 5. Mobile card processing 6. Daily living assistance 7. Daily travel booking and assistance
 6Family accommodation
6Family accommodation
1. Based on 2 persons/month 2. Provide accommodation assistance to overseas personnel 5CAR-T therapy
making a treatment plan
5CAR-T therapy
making a treatment plan
1.CART cell technology Services 4Medical service
4Medical service
1. Designated hospitals are green 2. Assist in arranging examinations, hospitalization and treatment 3. Interpreter accompanied by hospital 4. Assist in issuing bills
Top hospitals for CAR T-Cell therapy in China
The Second Affiliated Hospital of Zhejiang University School of Medicine
Multidisciplinary Precise Diagnosis and Treatment of Adult Lymphoma The precise diagnosis and ...
The University of Hong Kong-Shenzhen Hospital
It’s a large, comprehensive public hospital fully invested by the Shenzhen Municipal Government a...
Hematology Department of Tongji Hospital of Tongji University
Rich clinical experience in the diagnosis and treatment of various common and difficult diseases ...
Department of Hematology, Beijing Chao-yang Hospital, Capital Medical University
The Respiratory Disease in the Hospital is the national key specialty under the Ministry of Educa...
Peking University People's Hospital, Peking University Institute of Hematology
One of the largest comprehensive institutes of hematology in China Peking University Institute...
Blood Diseases Hospital Affiliated to Chinese Academy of Medical Sciences (Institute of Hematology)
Consecutively ranked No.1 for 14 years from 2010 to 2023 in China Hospital Specialty Reputation L...
Jiahui Health Ecosystem
Jiahui Health is an international-standard medical and healthcare ecosystem. As an innovative and...
Beijing Boren Hospital
Beijing Boren Hospital is a non-governmental hospital committed to medical technique, humane conc...
Beijing United Family Hospital
Beijing United Family Hospital (BJU) has been offering premium care to hundreds of thousands of e...
Expert Team

Lihong An
Expertise Specializes in the treatment of refractory and relapsed acute lymphoblastic leukemia/my...
Read More
Defeng Zhao
He holds a Master's degree from the Second Military Medical University. He has been engaged in cl...
Read More
Shuangyou Liu
She holds a Doctor of Medicine from Heidelberg University, Germany. She has primarily been engage...
Read More
Chunrong Tong
She is the leader of the Immunology and Targeted Therapy discipline at the Gobroad Medical (Hemat...
Read More
Lugui Qiu
Director, Lymphoma Diagnosis and Treatment Center, Blood Diseases Hospital Affiliated to Chinese ...
Read More
Chong Wang
He graduated from the Medical School of Zhengzhou University with a master's degree in clinical m...
Read More
Zhen Cai
CAI Zhen, female, professor, chief physician, doctoral supervisor. He is currently the director o...
Read More
Rui hua Mi
Mi Ruihua, Master, Deputy Chief Physician, Hematology Department, Henan Cancer Hospital. He speci...
Read More
Xin LI
Main interests: Basic research on multiple myeloma, clinical research on hematological diseases r...
Read More
Bing Chen
Chen Bing, female, chief physician, doctoral supervisor. He has been engaged in hematology clinic...
Read More










