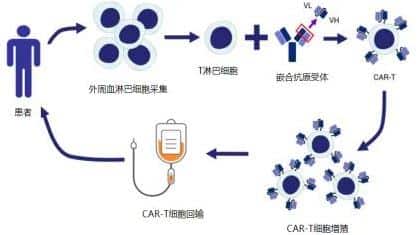
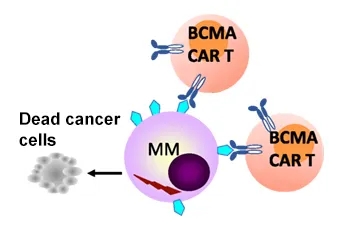
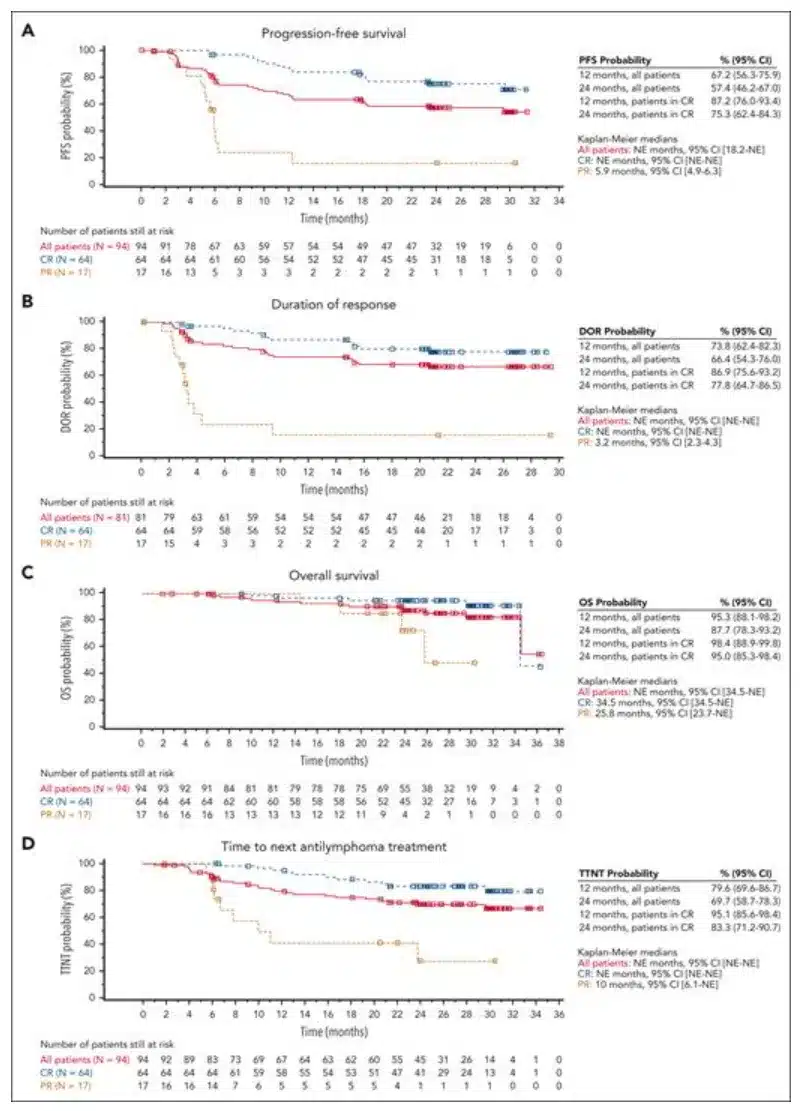
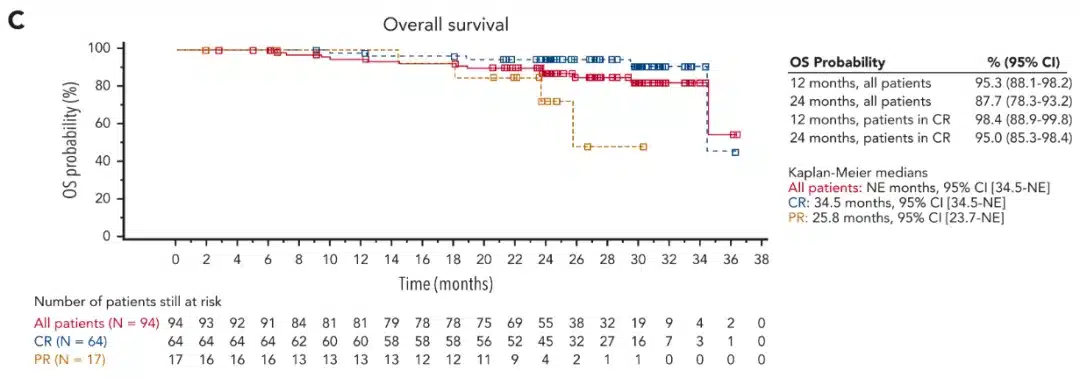
Lymphoma

Recently, the Department of Hematology at the University Hospital of Rennes in France published a journal review titled “Remission After CAR-T Cell Therapy: Can Lymphoma Patients Return to a Normal Life?” in the medical journal HemaSphere.

Recently, The Lancet Haematology published the results of a phase 1 clinical trial indicating that CD30-targeted CAR-T cell therapy (ATLCAR.CD30) has the potential to serve as a consolidation treatment for high-risk Hodgkin lymphoma patients after autologous HSCT.

Primary central nervous system lymphoma (PCNSL) is a central nervous system-confined non-Hodgkin lymphoma (NHL), with the pathological diagnosis being mainly diffuse large B-cell lymphoma.

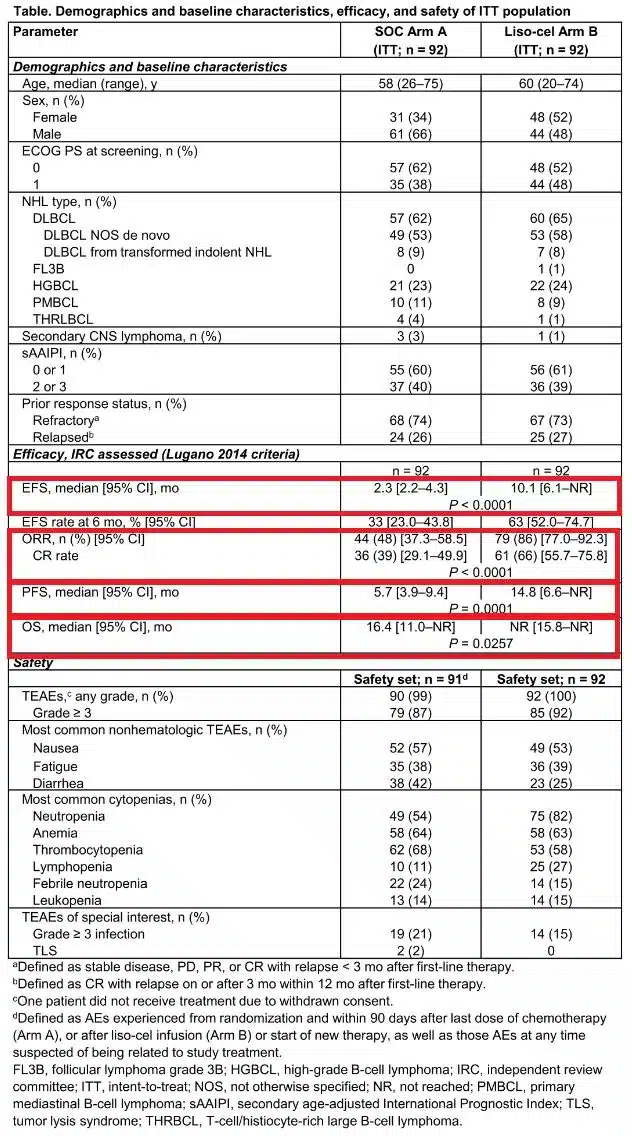
On May 30, 2024, Bristol Myers Squibb announced that the U.S. FDA has approved the expanded indication of its CAR-T therapy Breyanzi (lisocabtagene maraleucel; liso-cel) for the treatment of adult patients with relapsed or refractory mantle cell lymphoma (MCL) who have received at least two prior lines of systemic therapy, including those who have been previously treated with a Bruton’s tyrosine kinase (BTK) inhibitor.

On Thursday, May 31, 2024, the U.S. Food and Drug Administration (FDA) approved Bristol Myers Squibb’s Breyanzi (lisocabtagene maraleucel) for the treatment of adult patients with relapsed or refractory mantle cell lymphoma (MCL), making this CD19-targeted cellular therapy the broadest approved for B-cell malignancies.

On May 16, 2024, the U.S. Food and Drug Administration (FDA) announced accelerated approval for the expanded indication of Breyanzi, Bristol Myers Squibb’s CAR-T cell therapy, for the treatment of adult patients with relapsed or refractory follicular lymphoma (FL) after two or more lines of systemic therapy.