
Yescarta (Axicabtagene ciloleucel, axi-cel) is an autologous anti-CD19 chimeric antigen receptor (CAR)-T cell therapy approved for relapsed/refractory (R/R) follicular lymphoma (FL).

Yescarta (Axicabtagene ciloleucel, axi-cel) is an autologous anti-CD19 chimeric antigen receptor (CAR)-T cell therapy approved for relapsed/refractory (R/R) follicular lymphoma (FL).

Follicular lymphoma (FL) is the most common indolent lymphoma and the second most common type of lymphoma globally, accounting for approximately 22% of newly diagnosed lymphomas worldwide. Currently, there is no standard treatment for relapsed/refractory FL.
On May 4, 2022 (Basel), Novartis announced that the European Commission has expanded the approval of Kymriah (tisagenlecleucel) to include the treatment of adult patients with relapsed or refractory follicular lymphoma (FL) after two or more lines of systemic therapy.

On December 12, 2021, at the 63rd American Society of Hematology (ASH) Annual Meeting, Novartis presented subgroup analysis data from the Phase 2 ELARA study with a median follow-up of 17 months.

On October 18, 2017, the U.S. FDA approved the CAR-T cell therapy Yescarta for the treatment of adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy, including patients with diffuse large B-cell lymphoma (DLBCL) not otherwise specified, high-grade B-cell lymphoma, and DLBCL arising from follicular lymphoma.

On May 27, 2022, Novartis announced on its official website that the U.S. FDA has granted accelerated approval for a new indication of its CAR-T cell therapy Kymriah (tisagenlecleucel) for the treatment of adult patients with relapsed or refractory (r/r) follicular lymphoma (FL) after two or more lines of systemic therapy.
On May 2, 2018, the U.S. FDA approved Novartis’ CAR-T cell therapy drug Kymriah (Tisagenlecleucel, CTL019) for a second indication, to treat adult patients with relapsed or refractory large B-cell lymphoma (DLBCL) after two or more lines of systemic therapy, including the most common form of non-Hodgkin lymphoma—diffuse large B-cell lymphoma (DLBCL)—as well as high-grade B-cell lymphoma and DLBCL arising from follicular lymphoma (FL).
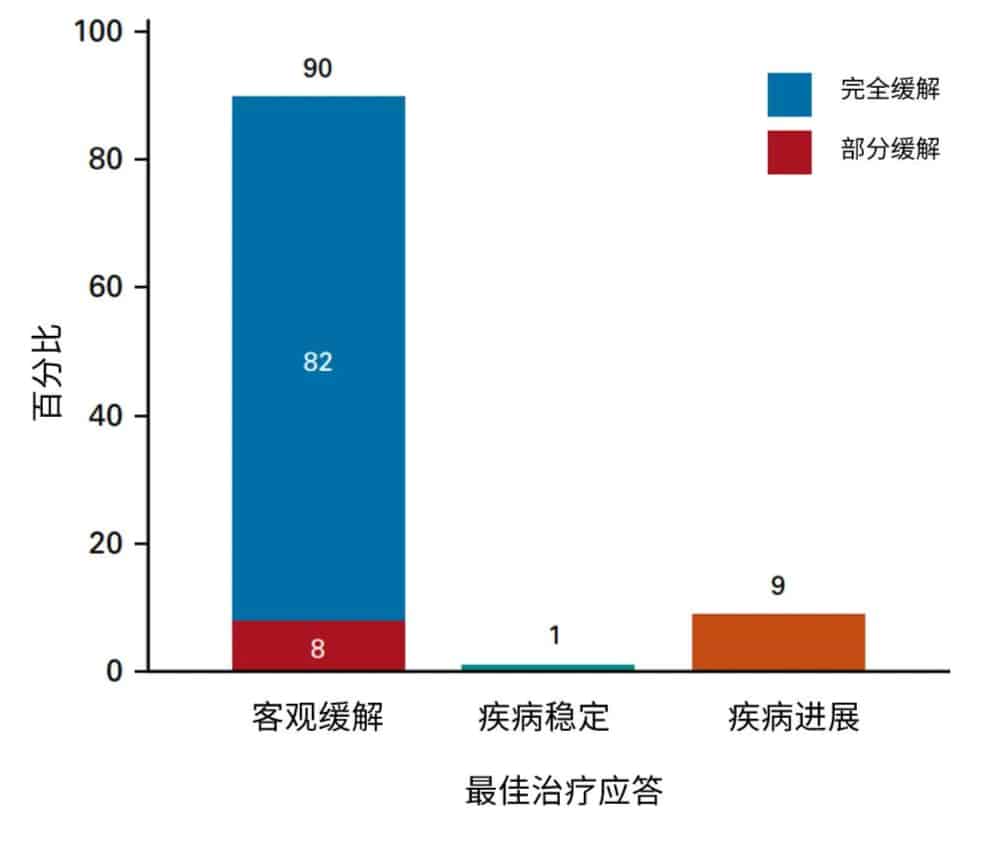
On February 8, 2023, JCO Online published the clinical results from the US Lymphoma CAR-T Alliance on Brexucabtagene Autoleucel (Tecartus, brexu-cel) as a standard treatment for relapsed/refractory mantle cell lymphoma. Let’s take a look with the editor.
By using our site, you agree to our Terms and Conditions and Privacy Policy.Advanced Medicine In China does not provide medical advice, diagnosis, or treatment. The information provided on this site is designed to support, not replace, the relationship that exists between a patient/site visitor and his/her existing physician.
© Copyright 2023 Advanced Medicine In China. All rights reserved.