Lymphoma

On April 1, 2024, the US Food and Drug Administration (FDA) granted approval to Kite, a Gilead Sciences subsidiary, for Yescarta (Axicabtagene Ciloleucel), a CAR-T cell therapy, for the treatment of adult patients with large B-cell lymphoma (LBCL) who are refractory to first-line chemotherapy immunotherapy or experience recurrence within 12 months of first-line chemotherapy immunotherapy.

On March 5, 2021, Gilead Sciences announced that the U.S. Food and Drug Administration (FDA) has granted accelerated approval to Yescarta (axicabtagene ciloleucel), a groundbreaking treatment for adult patients with relapsed or refractory follicular lymphoma (FL) who have undergone at least two systemic therapies.

On March 5, 2021, the U.S. Food and Drug Administration (FDA) granted accelerated approval to Yescarta (axicabtagene ciloleucel) for the treatment of adult patients with relapsed or refractory follicular lymphoma (FL) who have received at least two prior systemic therapies.

Yescarta (Axicabtagene Ciloleucel), a revolutionary CAR-T cell therapy, has garnered significant attention since its FDA approval in 2017. This article aims to provide a comprehensive overview of Yescarta, including its FDA approval history, indications, efficacy, safety profile, and potential future developments.

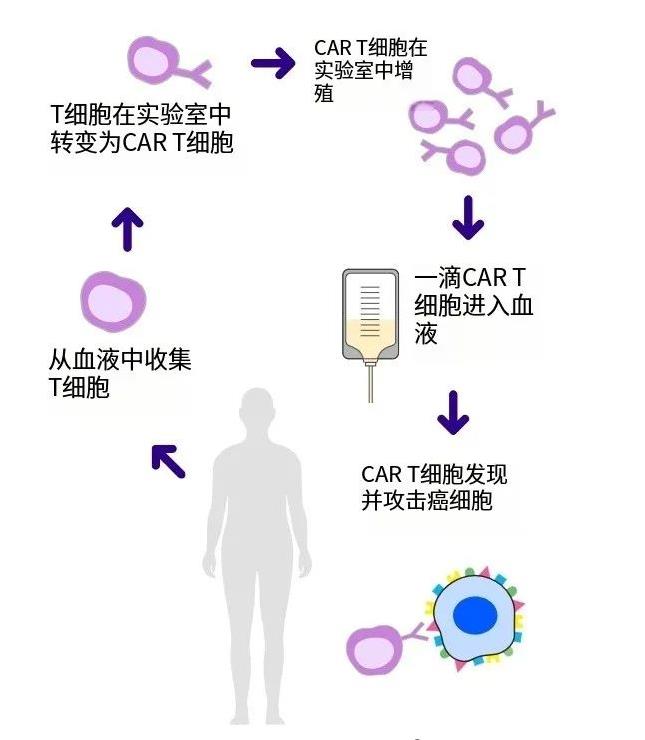
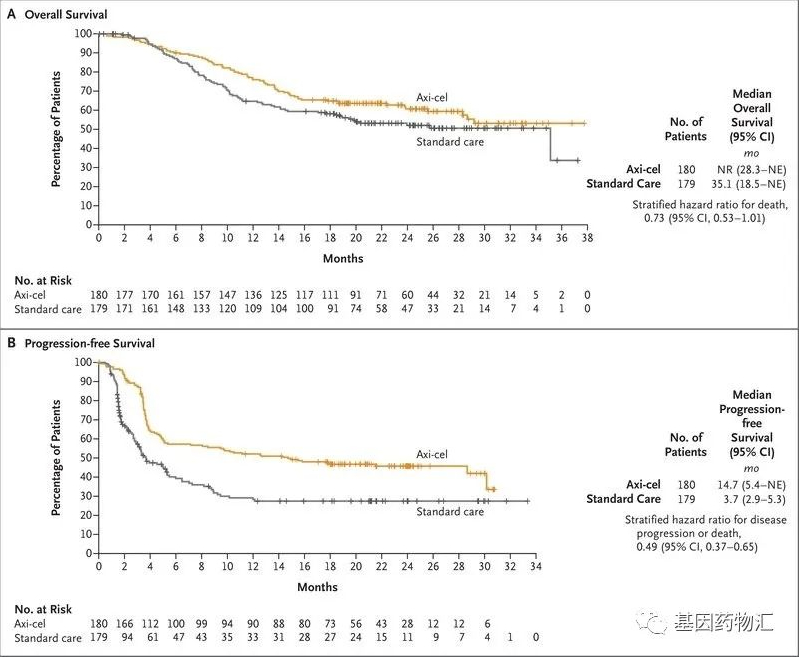
Yescarta (Axicabtagene ciloleucel, Axi-cel) has revolutionized the treatment landscape for lymphoma since its initial approval by the US FDA on October 18, 2017. As the world’s first chimeric antigen receptor T-cell (CAR-T) therapy for adult patients with relapsed or refractory large B-cell lymphoma (R/R LBCL) who have received two or more lines of systemic therapy, Yescarta has ushered in a new era in lymphoma treatment.