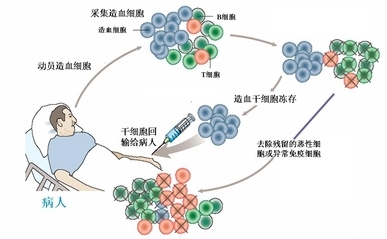
Lymphoma

Tecartus (brexucabtagene autoleucel), developed by Kite Pharma (now part of Gilead Sciences), is one such therapy that has gained significant attention for its efficacy in treating certain types of lymphoma and leukemia.

On July 24, 2020, the US Food and Drug Administration (FDA) granted accelerated approval to Tecartus (brexucabtagene autoleucel), a groundbreaking CAR-T cell therapy, for the treatment of adult patients with relapsed or refractory mantle cell lymphoma (MCL). Subsequently, on October 1, 2021, the FDA expanded the approval of Tecartus to include the treatment of adult patients with relapsed or refractory precursor B-cell acute lymphoblastic leukemia (ALL).

**Chinese Medical Team Research: 64.6% Complete Remission Rate, Over Half of Patients Progression-Free for More Than Two Years! Targeted Therapy Combined with Chemotherapy is Safe and Effective as First-Line Treatment for Lymphoma** Peripheral T-cell lymphoma (PTCL) is a group of highly aggressive and heterogeneous diseases with poor prognosis. The standard CHOP regimen (cyclophosphamide, doxorubicin, vincristine, Read More

In early June 2024, the results of a Phase I clinical trial for the CD7-CAR-T product PA3-17 in the treatment of relapsed/refractory T-cell acute lymphoblastic leukemia/lymphoblastic lymphoma (r/r T-ALL/LBL) were presented at the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting.