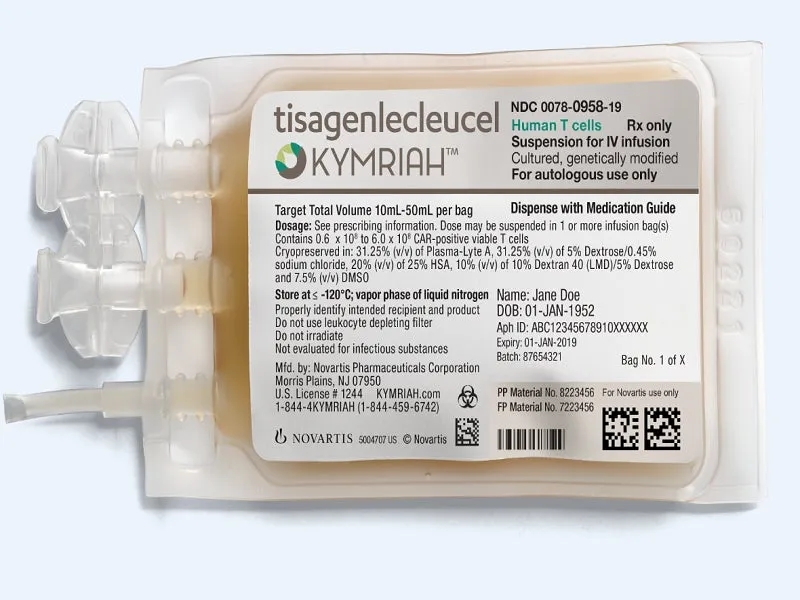
On May 27, 2022, the U.S. Food and Drug Administration (FDA) made a groundbreaking decision by approving Kymriah (tisagenlecleucel) for the treatment of adults with relapsed or refractory follicular lymphoma (FL) after two or more lines of systemic therapy.