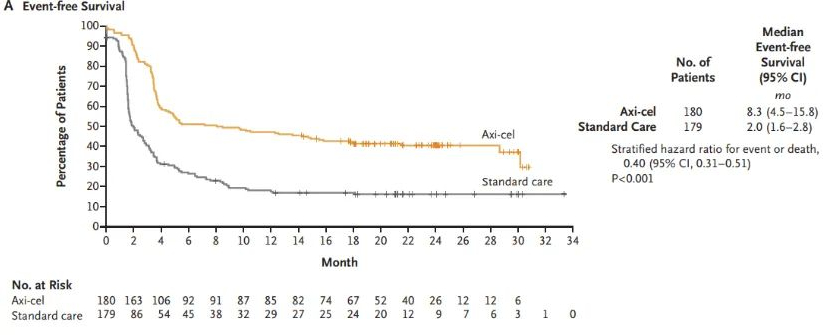
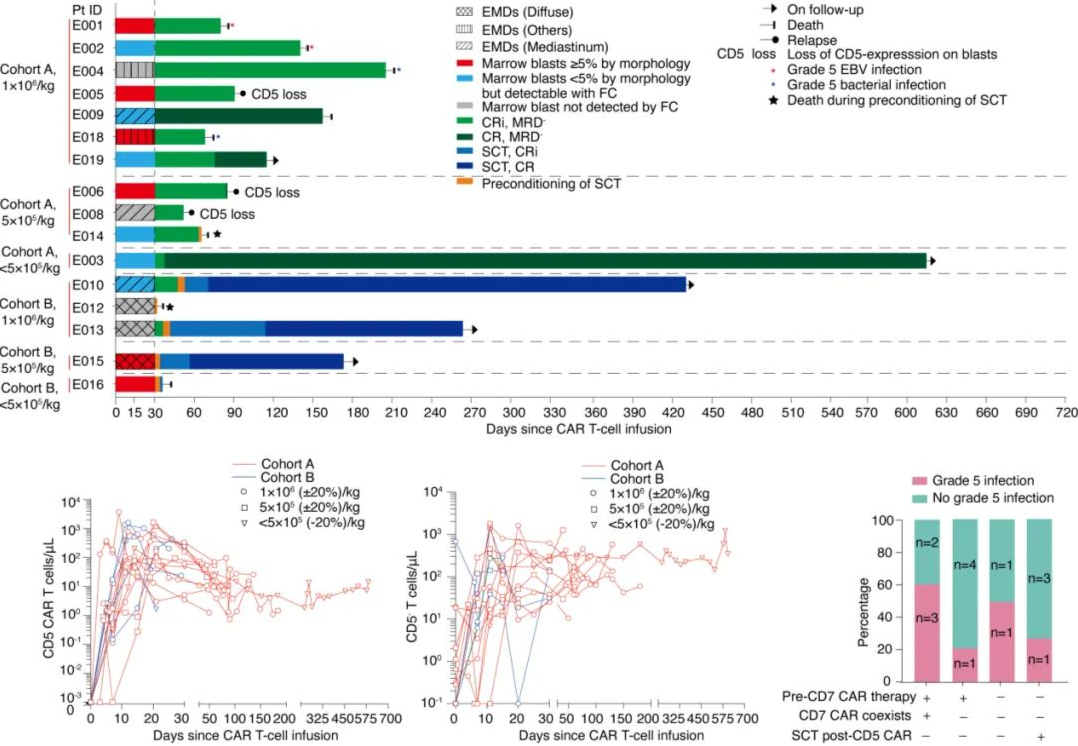
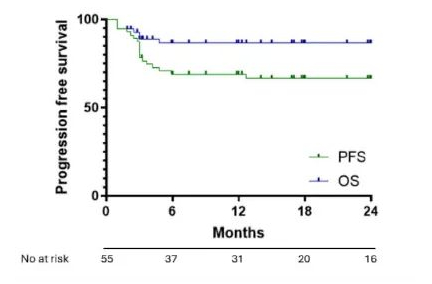
On April 1, 2022, Kite, a subsidiary of Gilead Sciences, announced that the U.S. Food and Drug Administration (FDA) has approved their CAR-T cell therapy product Yescarta® for the treatment of adult patients with large B-cell lymphoma (LBCL) that is refractory to first-line chemoimmunotherapy or relapses within 12 months after first-line chemoimmunotherapy.