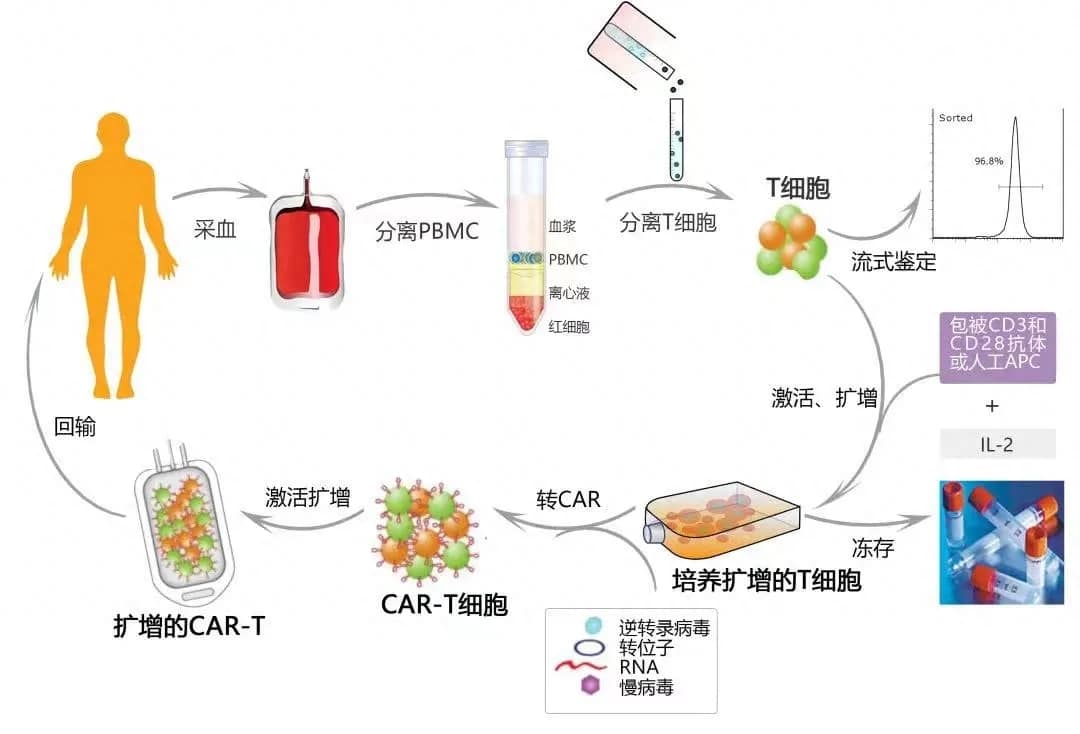
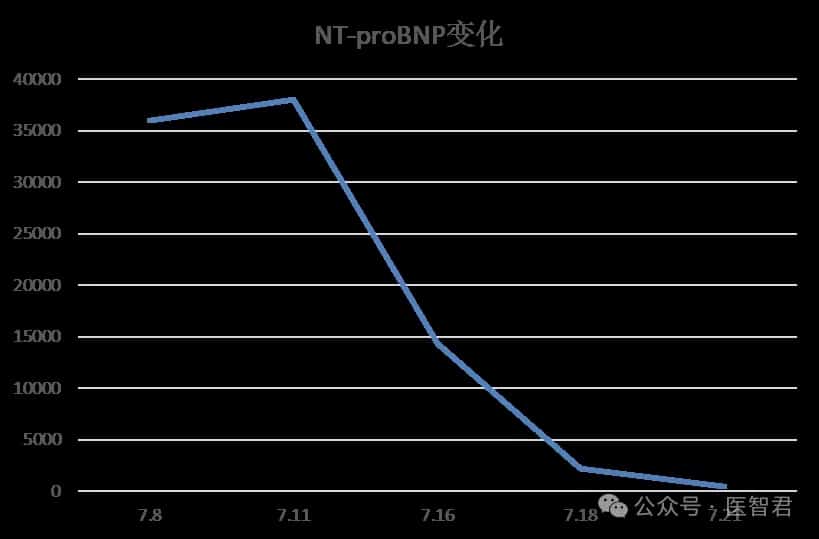
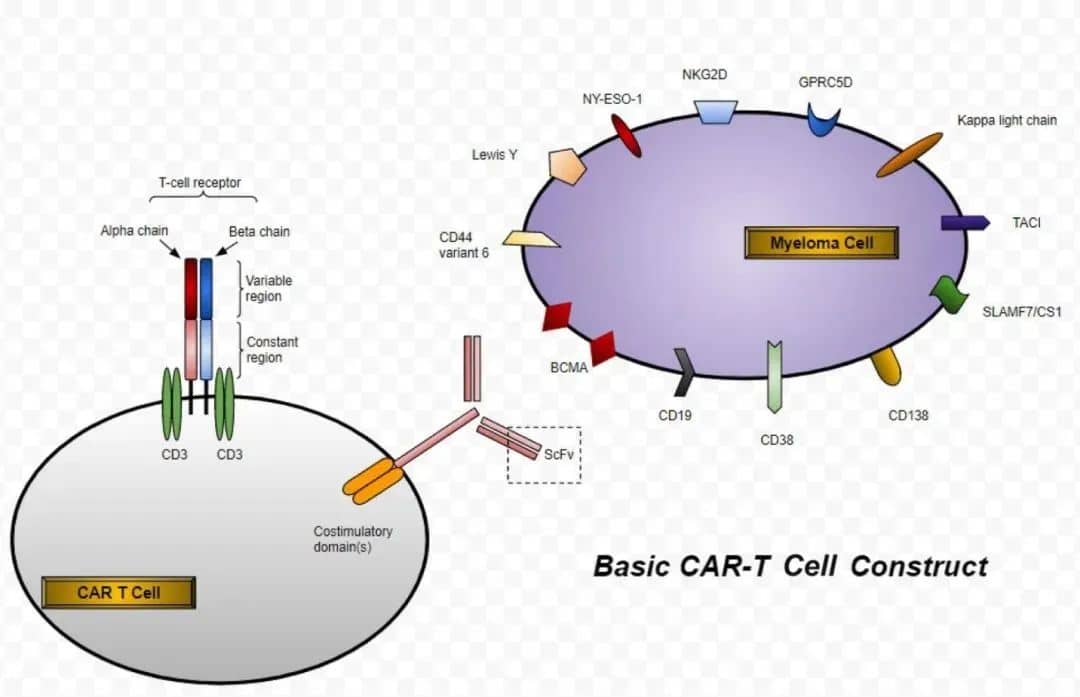
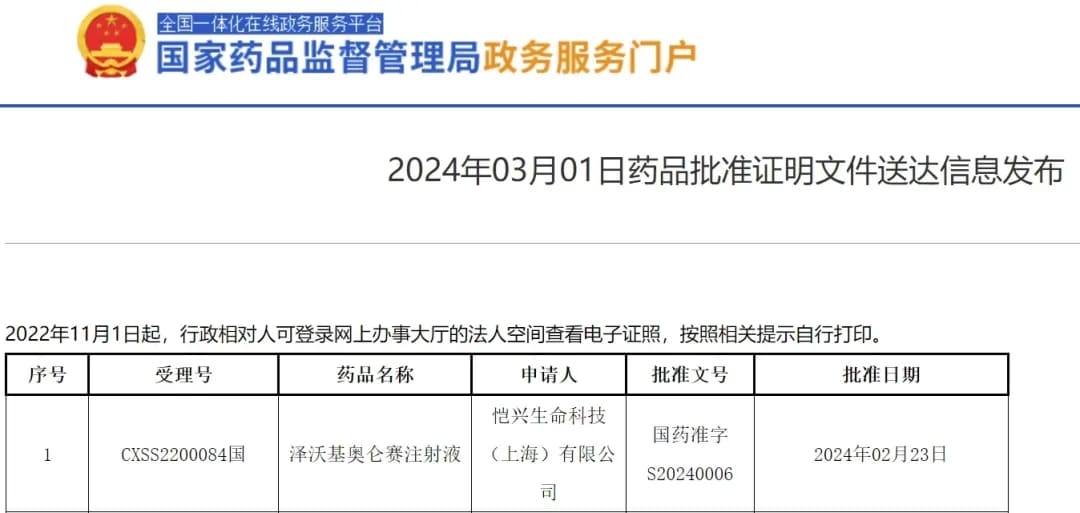
Recently, Legend Biotech (NASDAQ: LEGN) announced in Somerset, New Jersey, that the U.S. Food and Drug Administration (FDA) has approved CARVYKTI® (Ciltacabtagene Autoleucel, cilta-cel) for the treatment of adult patients with relapsed or refractory multiple myeloma (RRMM) who have received at least one prior line of therapy, including a proteasome inhibitor (PI) and an immunomodulatory agent (IMiD), and are refractory to lenalidomide1.