Myeloma
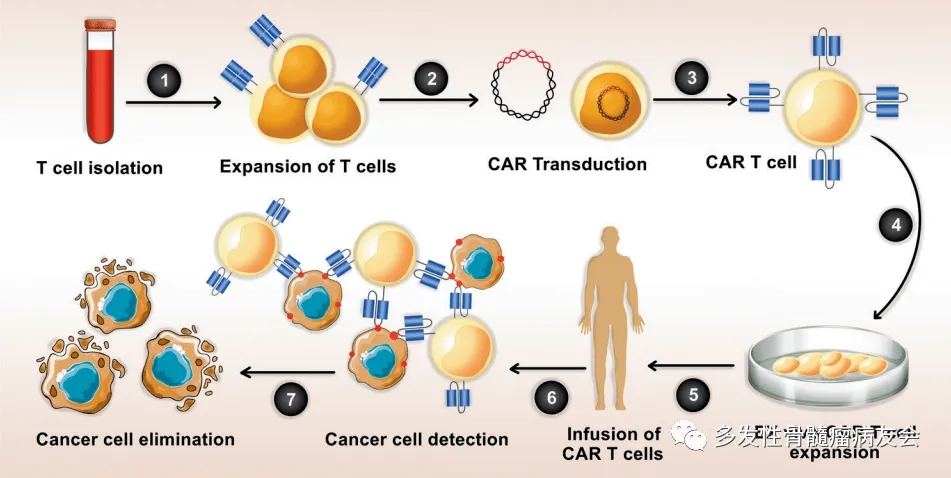
CAR-T cell therapy (Chimeric Antigen Receptor T-cell therapy) is a form of cellular immunotherapy that involves genetically modifying a patient’s T cells to express a specific chimeric antigen receptor (CAR), inducing an anti-tumor response.
March 15,2024,the FDA Oncologic Drugs Advisory Committee (ODAC) held a full-day meeting to review the benefit/risk profiles of Abecma from BMS/Bluebird Bio and Carvykti from Johnson & Johnson/Legend Biotech, two BCMA CAR-T therapies for frontline treatment.

🩸 Breakthrough in Multiple Myeloma Treatment! 🩺 🔬Targeting GPRC5D with CAR-T Cells shows immense potential in treating relapsed or refractory multiple myeloma patients. Multiple myeloma, a malignant disease characterized by clonal plasma cell proliferation in the bone marrow, has remained incurable despite substantial progress in treatment methods such as systemic chemotherapy, radiation therapy, and hematopoietic Read More
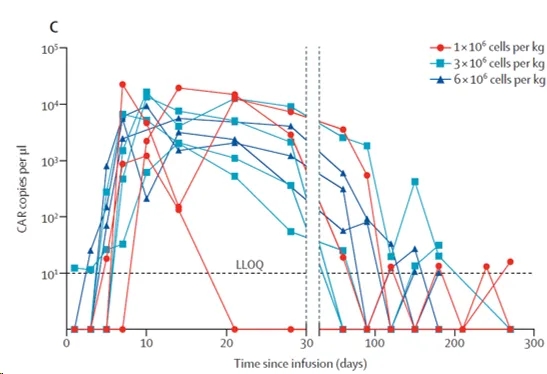
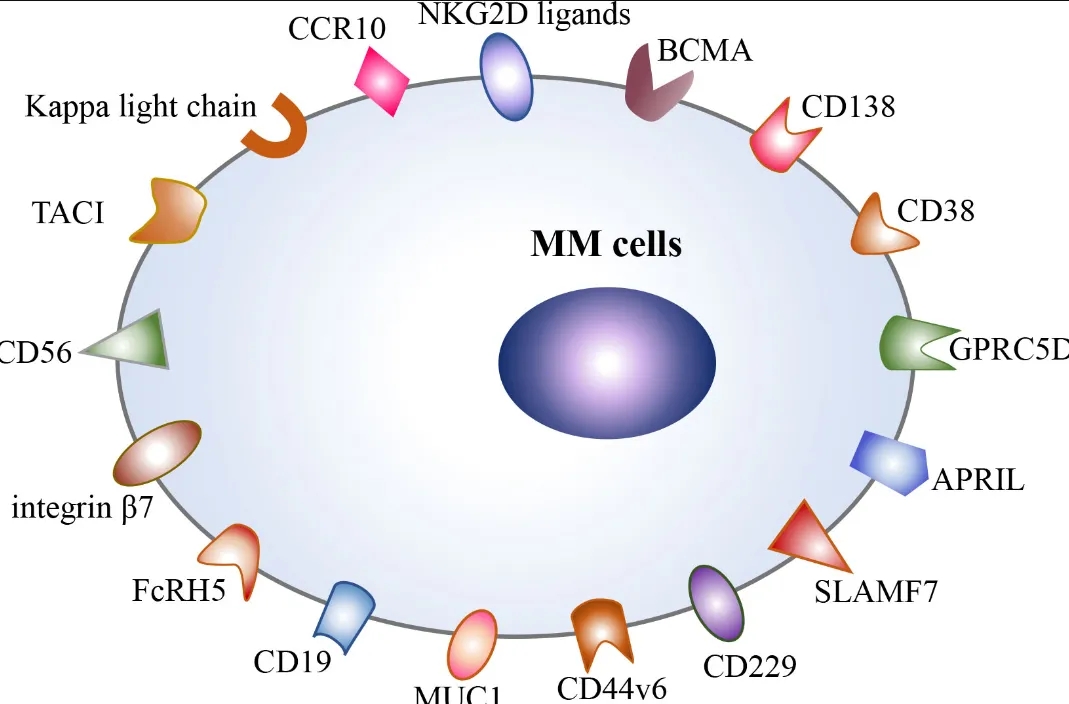
Chimeric antigen receptor (CAR) T-cell therapy targeting B-cell maturation antigen (BCMA) has shown activity in treating relapsed or refractory multiple myeloma
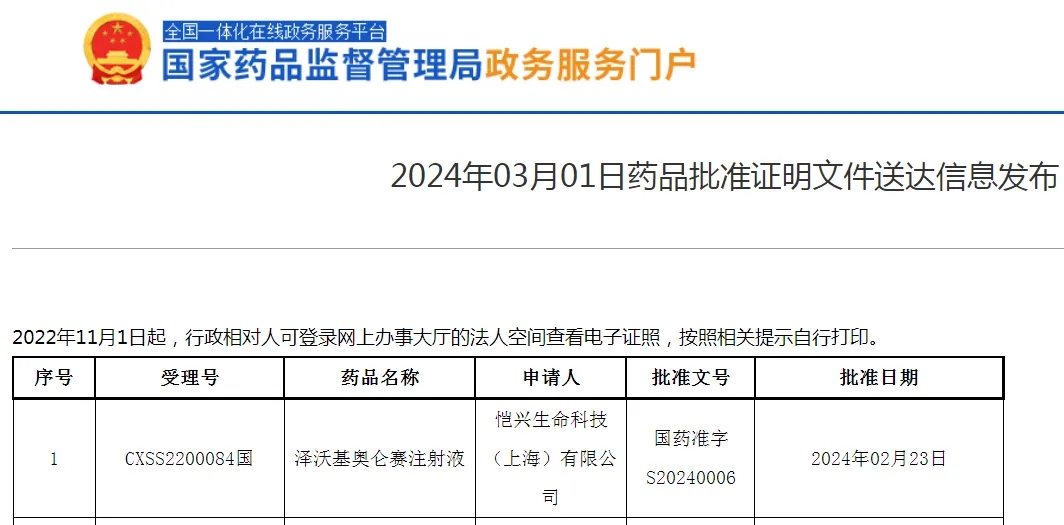
Zevorcabtagene autoleucel is the fifth CAR-T cell therapy approved for marketing in China, following FOSUN Kite’s Axicabtagene Ciloleucel Injection, JW THERAPEUTICS’ Relmacabtagene autoleucel, IASO Bio’s Equecabtagene Autoleucel, and Juventas’ Inaticabtagene Autoleucel Injection.
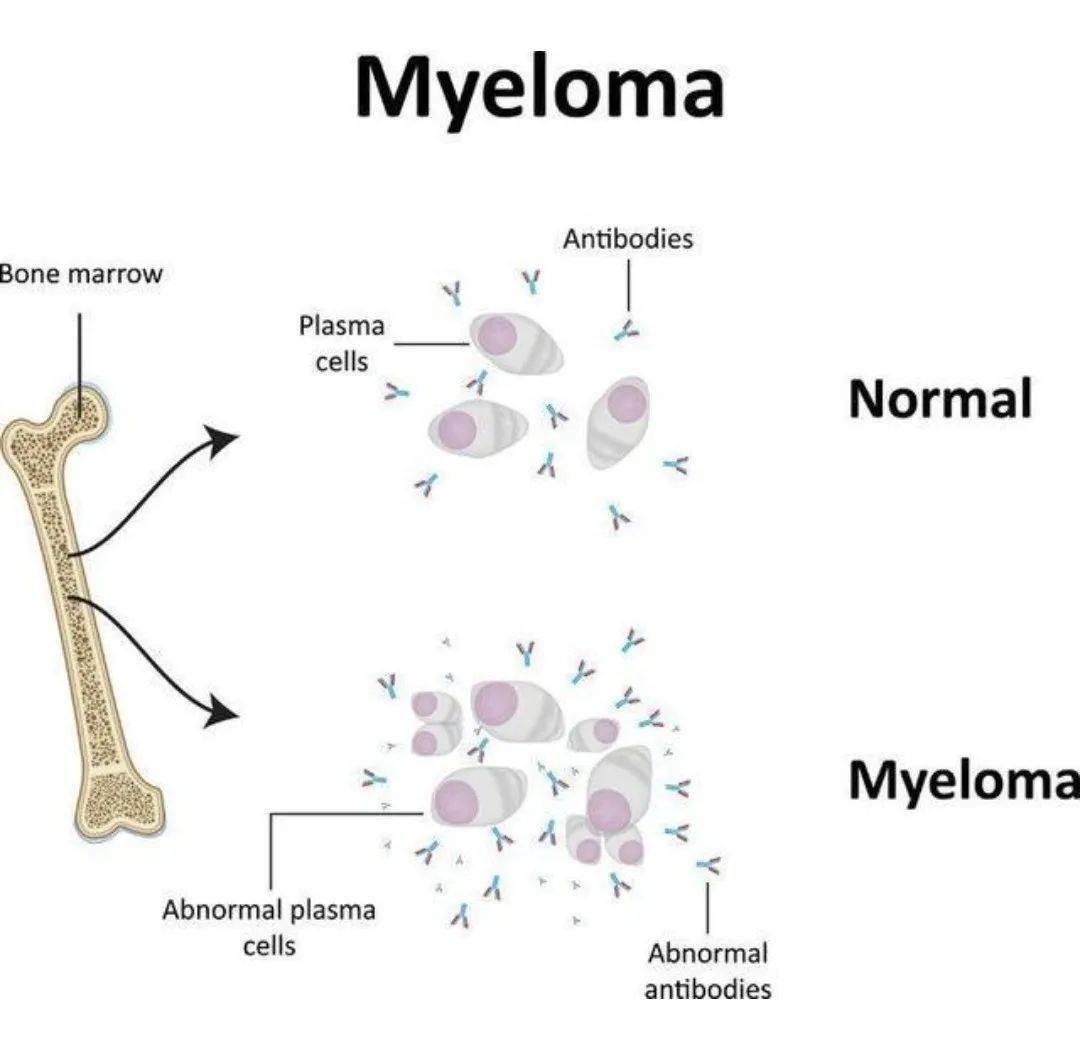
In recent years, clinical trials have demonstrated the remarkable efficacy of CAR-T therapy in relapsed/refractory MM (RRMM). Currently, two BCMA-targeted CAR-T therapies, idecabtagene vicleucel (ide-cel; trade name: Abecma) and ciltacabtagene autoleucel (cilta-cel; trade name: Carvykti), have been approved by the FDA in 2021 and 2022, respectively, for the treatment of RRMM in patients who have received at least five prior lines of therapy.