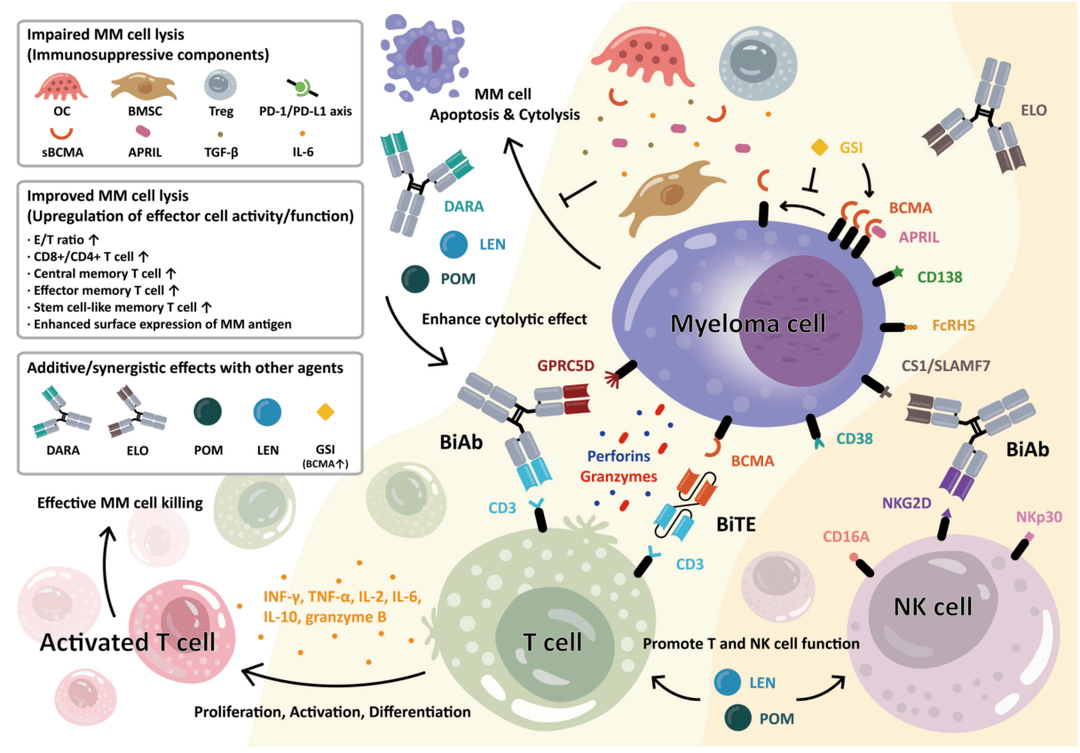
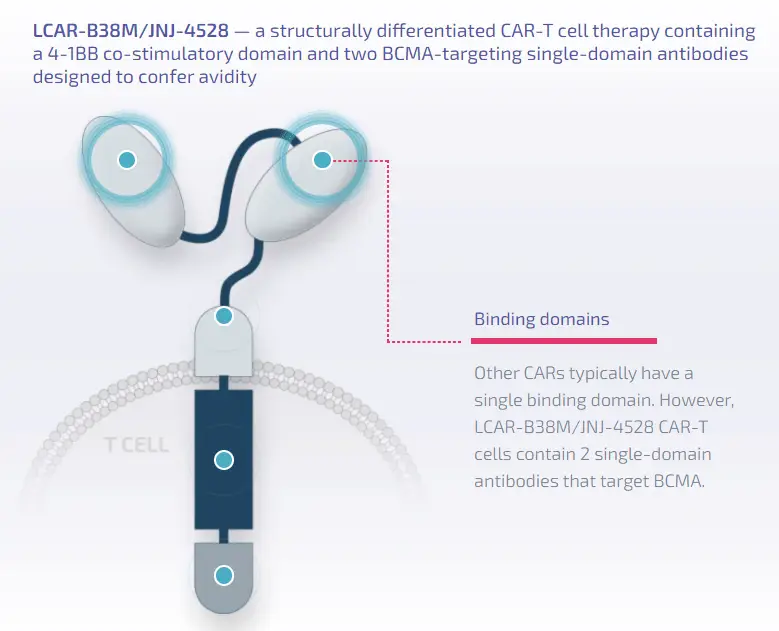
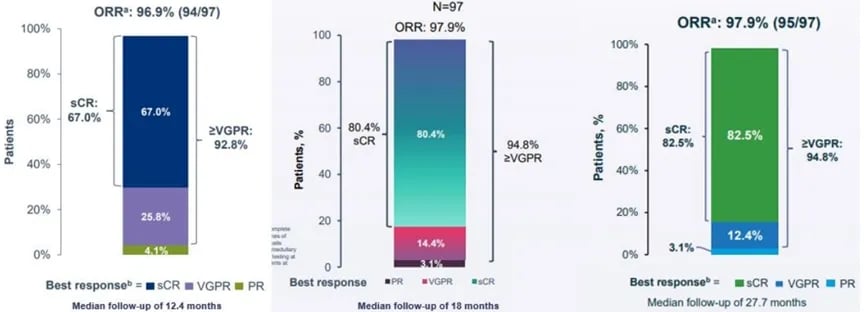
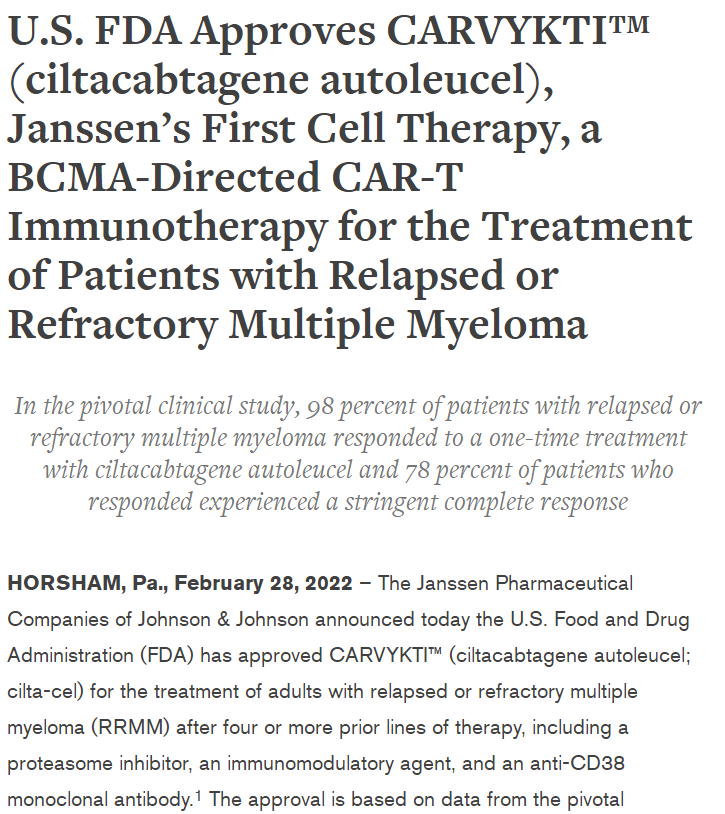
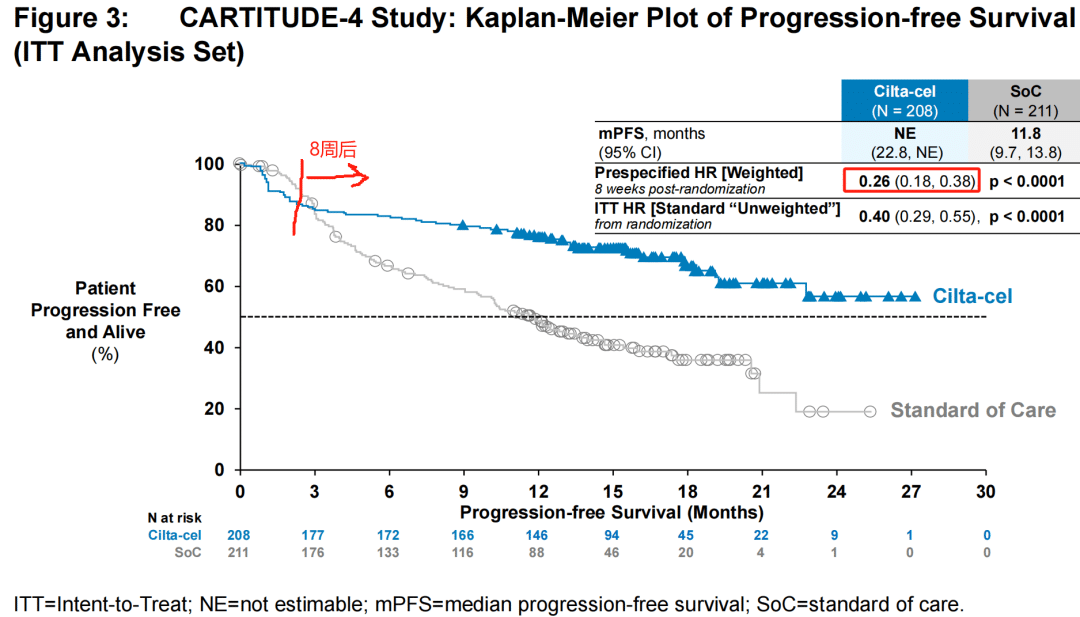
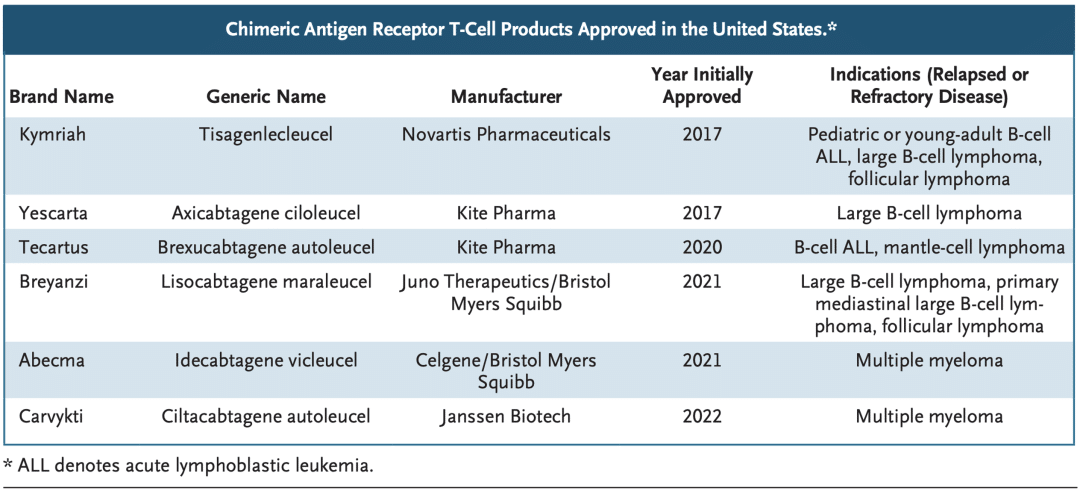
On March 2-3, 2024, the 7th Beijing Thrombosis and Hemostasis Conference and the 5th Beijing Hematologic Malignancies and Immunology Summit, hosted by the National Clinical Research Center for Blood Diseases, Institute of Hematology & Blood Diseases Hospital, Peking Union Medical College & Chinese Academy of Medical Sciences, was successfully held in Beijing.