Hematological Neoplasms

### China’s Breakthrough in CAR-T Therapy for CNS Lymphoma In a pioneering effort to improve outcomes for patients with refractory/relapsed central nervous system lymphoma (CNSL), a multi-center retrospective study led by Professor Jianqing Mi from Ruijin Hospital, Shanghai Jiaotong University School of Medicine, has showcased the impressive real-world effectiveness of Relmacabtagene Autoleucel (Relma-cel). This study, Read More

As of May 1, 2018, the FDA has approved Novartis’ CD19-targeted CAR-T therapy tisagenlecleucel (Kymriah) for the treatment of adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy, including diffuse large B-cell lymphoma (DLBCL), high-grade B-cell lymphoma, and DLBCL arising from follicular lymphoma.
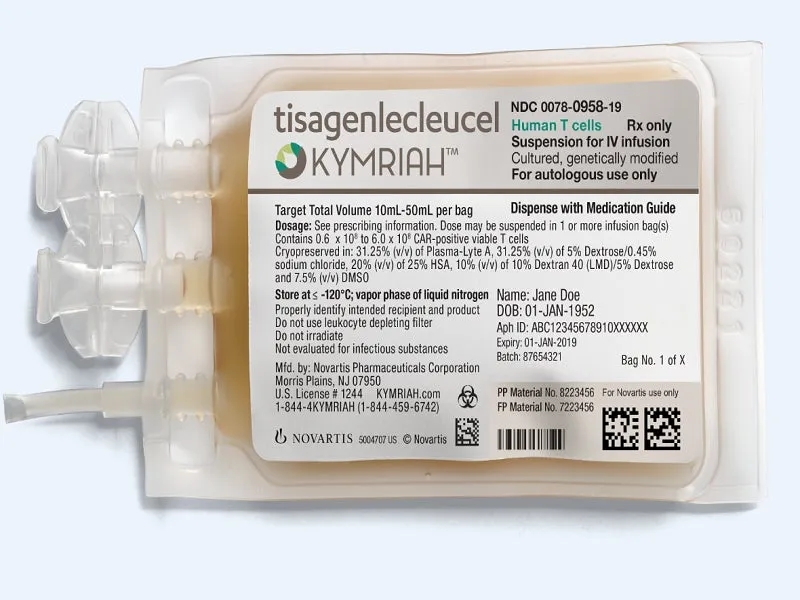
Kymriah (tisagenlecleucel) is a groundbreaking chimeric antigen receptor T-cell (CAR-T) therapy developed by Novartis. It represents a novel approach to cancer treatment by using the patient’s own genetically modified T-cells to target and kill cancer cells.

Axicabtagene ciloleucel (axi-cel, Yescarta) and tisagenlecleucel (tisa-cel, Kymriah) were the first two CAR-T products approved in Europe for the treatment of relapsed/refractory large B-cell lymphoma (R/R LBCL) patients, and they have accumulated some real-world experience.

Kite Pharma Yescarta (Axicabtagene Ciloleucel) Product Information Generic Name: Axicabtagene Ciloleucel Injection Brand Name: Yescarta Indications 1. Large B-Cell Lymphoma Yescarta is indicated for the treatment of: Adult patients with large B-cell lymphoma that is refractory to or relapses within 12 months after first-line chemoimmunotherapy. Adult patients with relapsed or refractory large B-cell lymphoma after Read More


