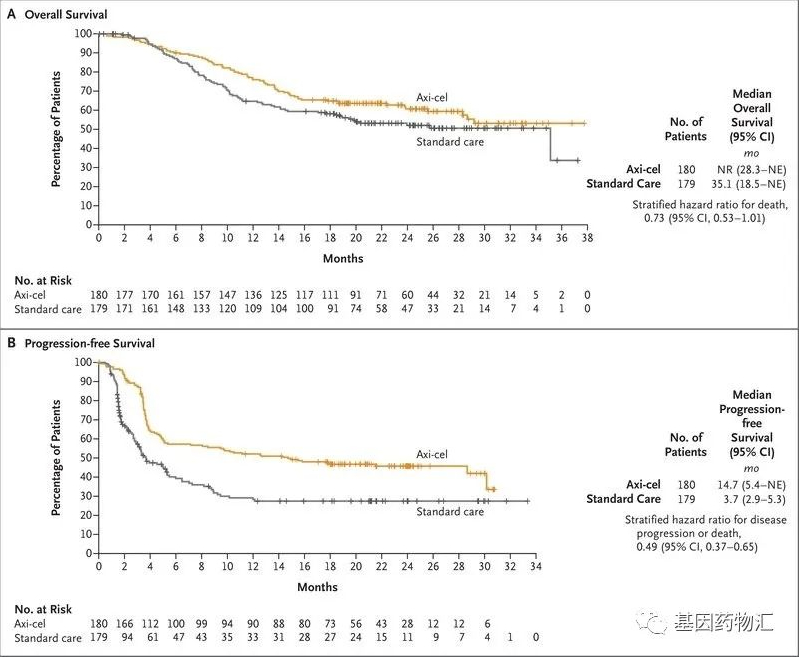
Interpretation of Hot Topics Conerning Mutiple Myeloma Patients: “What are the adverse reactions after CAR-T cell reinfusion?” Process Diagram for Myeloma Patients Receiving CAR-T Therapy RRMM(Relapsed/Refractory Multiple Myeloma) Unveiling how the “million-dollar anti-cancer shot” saves patients with recurrent and refractory bone tumors! Taking you through the entire process of CAR-T therapy. CAR-T细胞回输后有哪些不良反应? 骨髓瘤患者接受CAR-T治疗的流程图 权威专家解读之骨髓瘤全程管理 Expert Read More