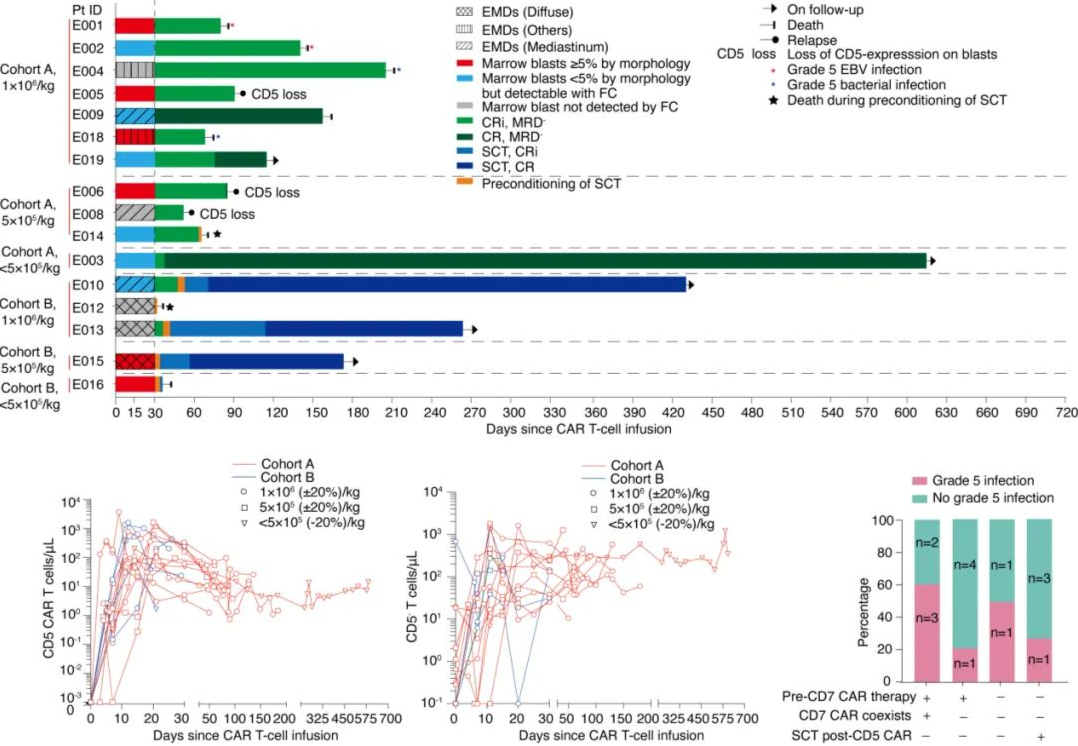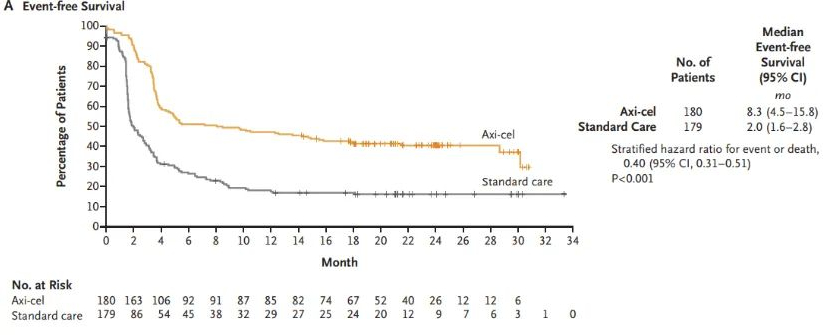
On October 17, 2022, exciting news came from the European Commission (EC). Based on the data from the ZUMA-7 study, the EC officially approved a new indication for Yescarta® for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) or high-grade B-cell lymphoma (HGBL) after one line of chemoimmunotherapy failed or within 12 months of first-line chemoimmunotherapy.