Hematological Neoplasms

Recently, the Department of Biological Therapy at the Tumor Medicine Department of the Chinese PLA General Hospital welcomed the return visit of one of the first CAR-T cell therapy patients who received treatment 10 years ago and achieved a cure.

Chimeric antigen receptor (CAR)-modified T cells have successfully treated B cell lymphomas, but applying a similar approach to TCLs is more challenging due to the complexity of targeting T cell antigens.

Recently, a review was published in Molecular Cancer by corresponding authors Prof. Hongbing Zhao from The First Affiliated Hospital of Xinxiang Medical University and Prof. Vivek P. Chavda from Lallubhai Motilal College of Pharmacy (India), and first authors Prof. Zoufang Huang from The First Affiliated Hospital of Gannan Medical University and Prof. Vivek P. Chavda. The review briefly summarized the development of CAR-T cell therapy for MCL. Key points are compiled here for reference.

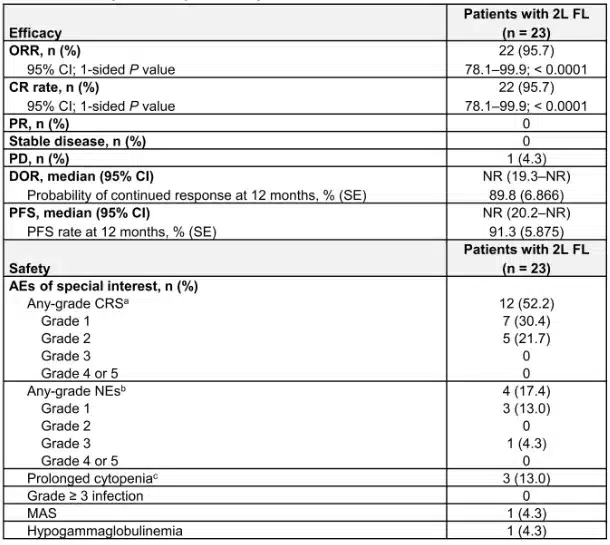
In October 18, 2017, the FDA approved Yescarta(Axicabtagene Ciloleucel), a CAR T-cell therapy, for the treatment of relapsed or refractory follicular lymphoma in adults after two or more prior lines of systemic therapy.

Lisocabtagene maraleucel (liso-cel) is an autologous CD19 4-1BB CAR-T cell product that has demonstrated high response rates and deep responses in treating R/R B-cell lymphomas.
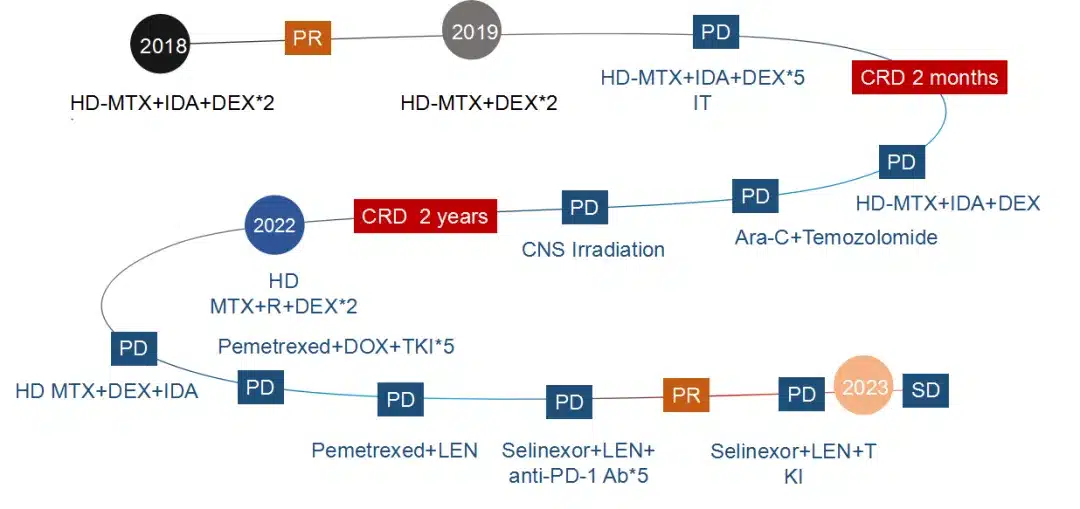
For relapsed/refractory (R/R) PCNSL, on the pathological level, the tumor cells are highly malignant and progress rapidly; on the patient level, the involvement of the brain is significant, leading to poorer function of other organs, and neurological function and physical condition often deteriorate rapidly.

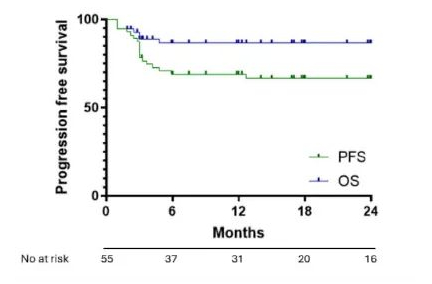
The American Journal of Hematology and Hemasphere recently published articles investigating the application of CAR-T in elderly DLBCL patients, one comparing CAR-T with immunochemotherapy in the elderly subgroup, and the other comparing CAR-T treatment in patients <70 years and ≥70 years.