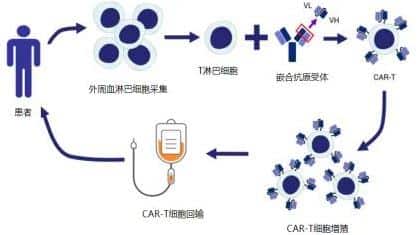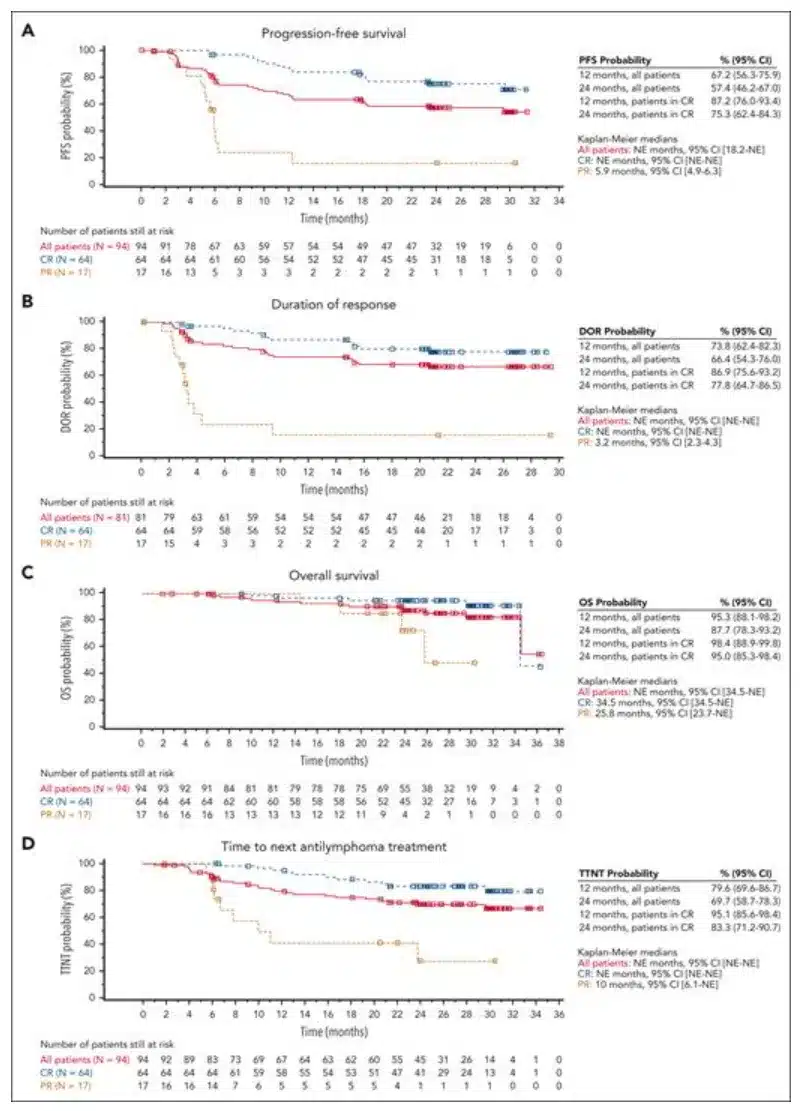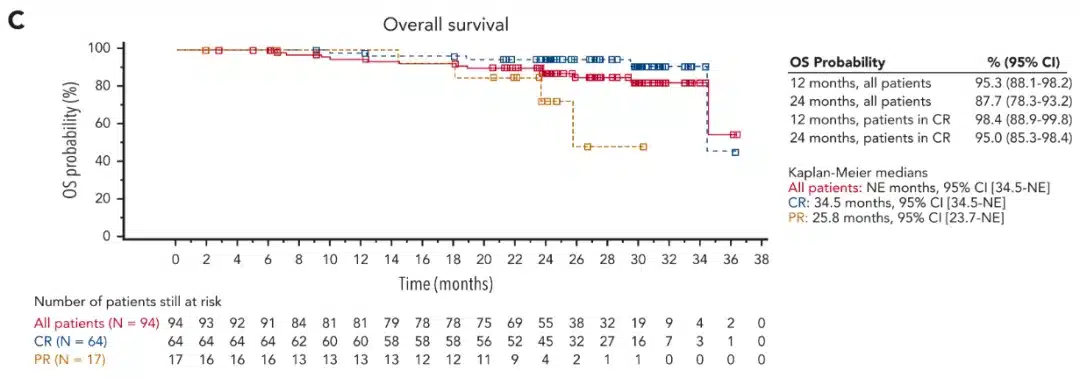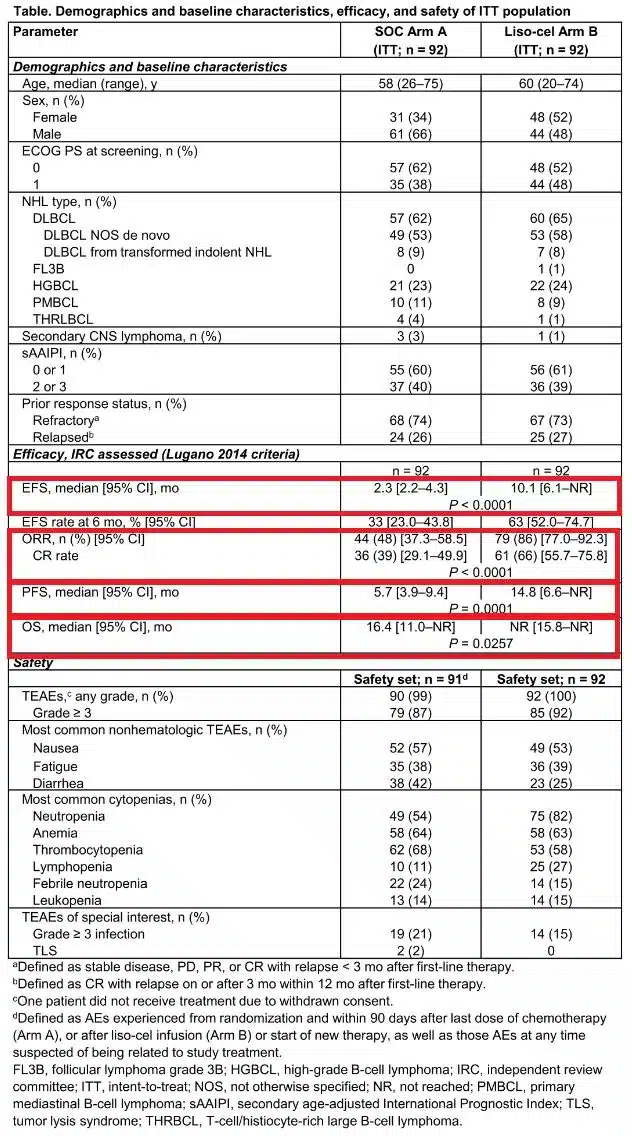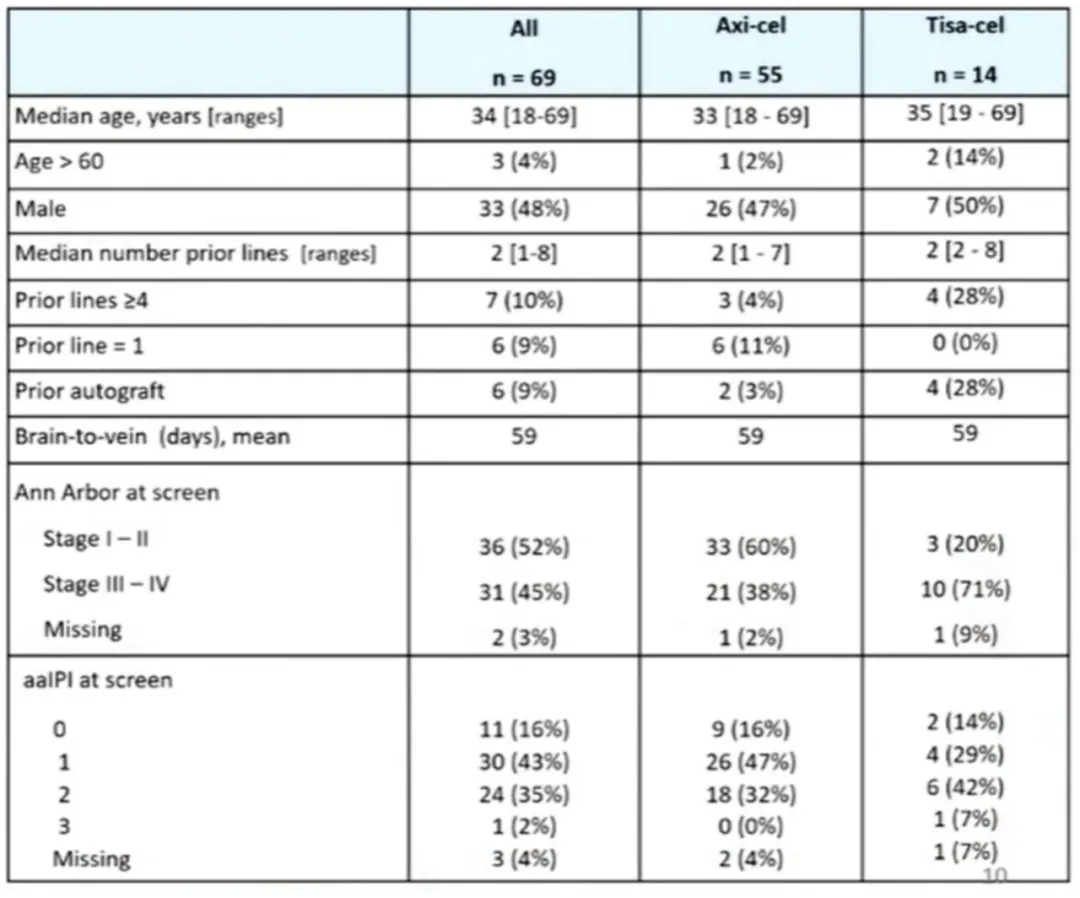
The editor has selected an “Oral Presentation” (S240)[2] to provide insights into the efficacy and toxicity of anti-CD19 autologous chimeric antigen receptor T (CAR-T) cell therapy in patients with relapsed or refractory primary mediastinal B-cell lymphoma (R/R PMBL), and to investigate factors associated with treatment outcomes.