Hematological Neoplasms

On May 16, 2024, the U.S. Food and Drug Administration (FDA) announced accelerated approval for the expanded indication of Breyanzi, Bristol Myers Squibb’s CAR-T cell therapy, for the treatment of adult patients with relapsed or refractory follicular lymphoma (FL) after two or more lines of systemic therapy.

Breyanzi was approved by the U.S. FDA on February 5, 2021, for the treatment of adult patients with relapsed or refractory large B-cell lymphoma (r/r LBCL) after two or more lines of systemic therapy, including diffuse large B-cell lymphoma (DLBCL) not otherwise specified, high-grade B-cell lymphoma, primary mediastinal large B-cell lymphoma, and follicular lymphoma grade 3B.

Breyanzi is a CD19-directed chimeric antigen receptor (CAR) T-cell immunotherapy developed by the American pharmaceutical company Bristol Myers Squibb (BMS) for the first-line treatment of adult patients with relapsed or refractory (r/r) large B-cell lymphoma (LBCL) after treatment.

22 Days from Desperation to Rebirth! Chinese CAR-T Therapy Creates Survival Miracle for Thai Multiple Myeloma Patient Subtitle: Fighting for Love! A story of miraculous rebirth after all treatment options failed for a late-stage multiple myeloma patient in Thailand, who underwent CAR-T therapy in China. Preface: When all treatment options had been exhausted, cancer progressed Read More

Breyanzi (lisocabtagene maraleucel), a CD19-directed chimeric antigen receptor T cell (CAR-T) therapy, developed by Bristol Myers Squibb (BMS), has made significant strides in the field of cancer treatment since its initial FDA approval in 2021.
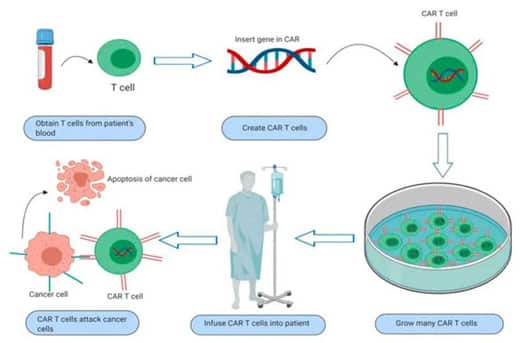
On July 24, 2020, the U.S. FDA announced accelerated approval of the CAR-T cell therapy Brexucabtagene Autoleucel (Tecartus) for the treatment of adult patients with relapsed or refractory mantle cell lymphoma (MCL).

Interpretation of Hot Topics Conerning Relapsed/Refractory Mutiple Myeloma Patients: What other treatment options are available for patients with relapsed Multiple Myeloma? How effective is the new drug treatment for the first relapse of Multiple Myeloma? What treatment options are available for initial relapse of multiple myeloma? What are the treatment goals for Relapsed/Refractory Multiple Myeloma Read More

On May 30, 2024, Bristol Myers Squibb (BMS) announced that the U.S. Food and Drug Administration (FDA) has approved Breyanzi (lisocabtagene maraleucel, liso-cel) for the treatment of adult patients with relapsed or refractory mantle cell lymphoma (MCL) who have previously received at least two lines of systemic therapy, including a Bruton’s tyrosine kinase (BTK) inhibitor.


