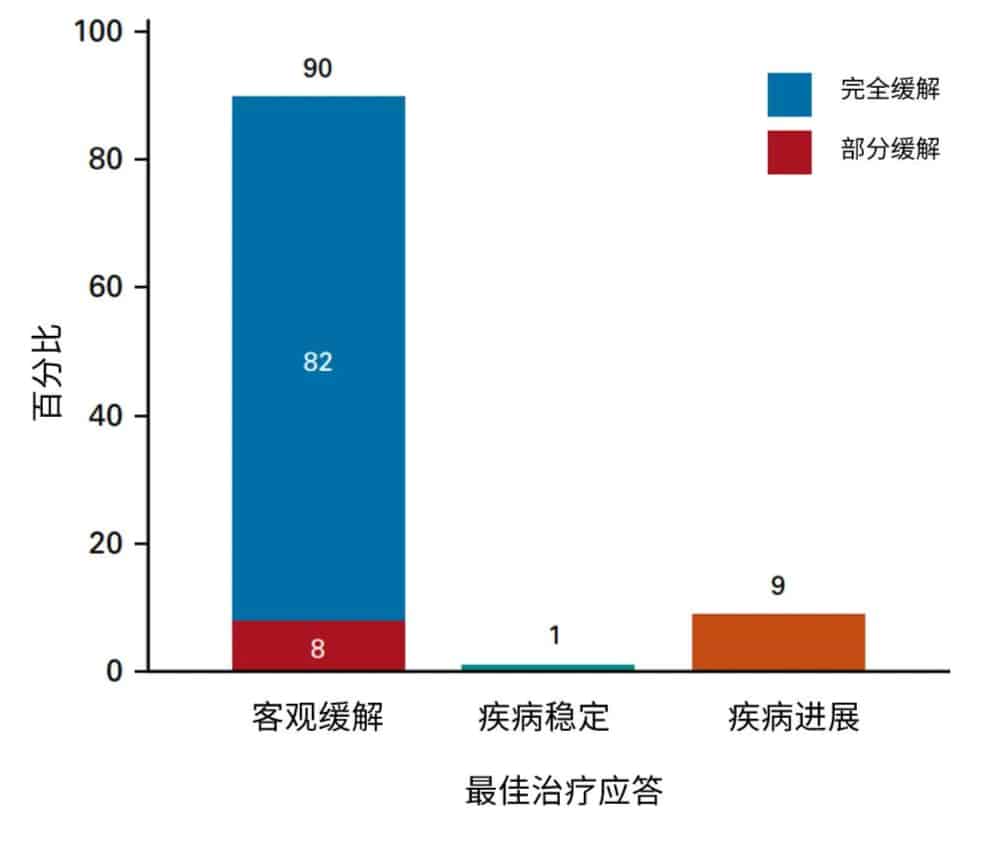
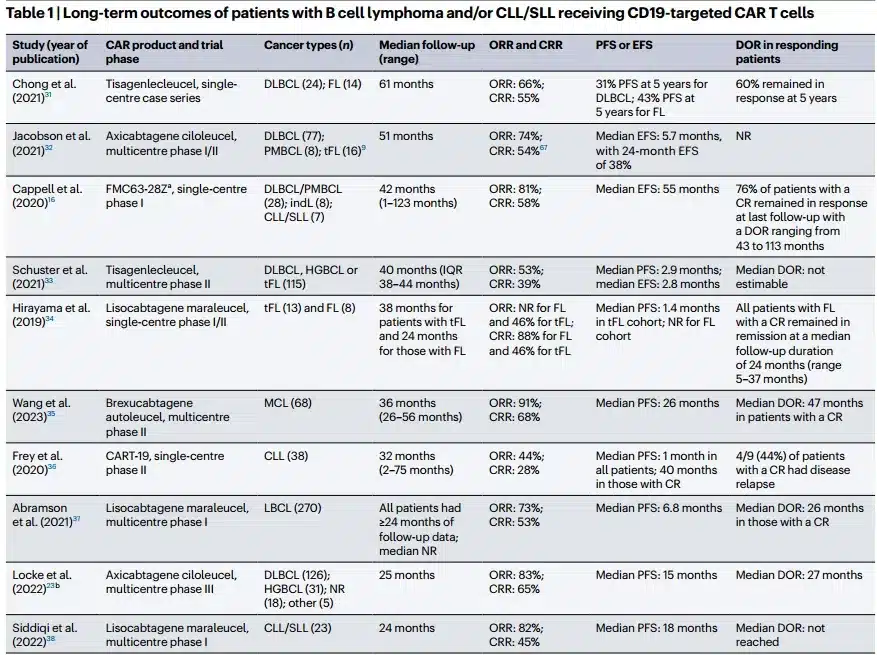
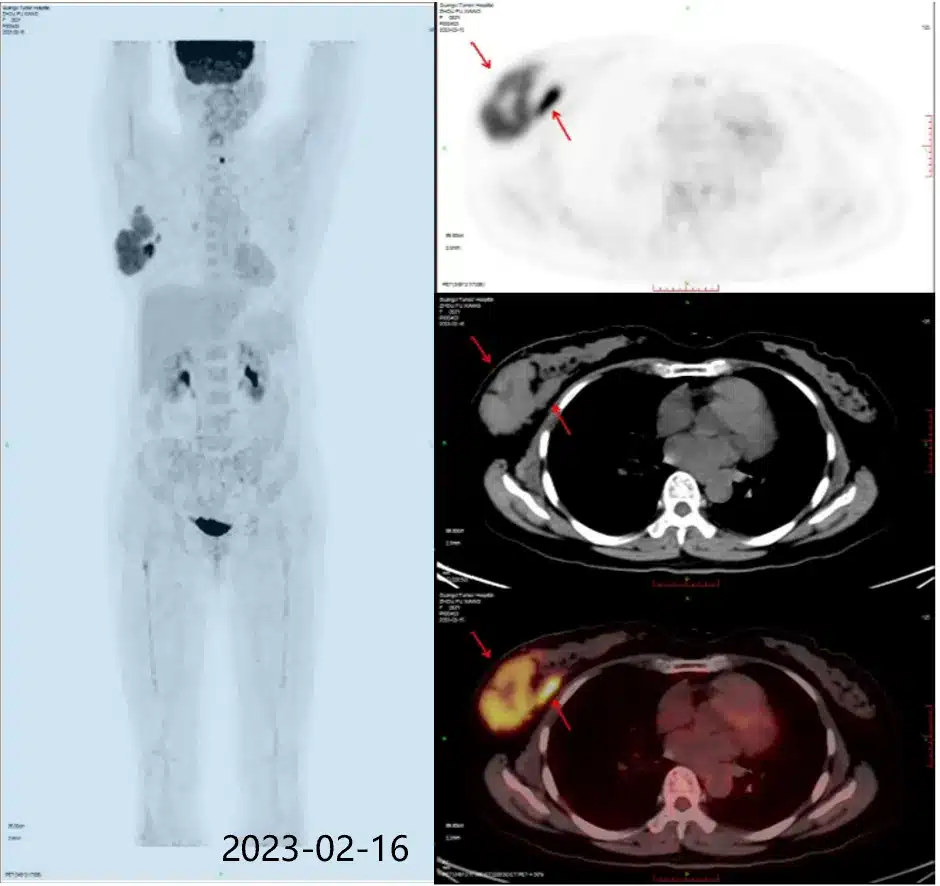
On October 18, 2017, the U.S. FDA approved the CAR-T cell therapy Yescarta for the treatment of adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy, including patients with diffuse large B-cell lymphoma (DLBCL) not otherwise specified, high-grade B-cell lymphoma, and DLBCL arising from follicular lymphoma.