Hematological Neoplasms

On May 15th, 2024 (local time), the U.S. Food and Drug Administration (FDA) announced the accelerated approval of Breyanzi (lisocabtagene maraleucel), Bristol Myers Squibb’s CAR-T therapy, for an expanded indication to treat adult patients with relapsed or refractory follicular lymphoma (FL) who have received at least two prior systemic therapies.

According to a retrospective study presented at the 2024 Transplantation & Cellular Therapy Meetings, a single infusion of brexucabtagene autoleucel (brexu-cel) achieved a higher rate of central nervous system (CNS) remission in patients with relapsed/refractory B-cell acute lymphoblastic leukemia (R/R B-ALL) and active CNS involvement.

The American Association for Cancer Research (AACR) Annual Meeting is the most influential oncology scientific event globally. From population science and prevention, to cancer biology, translational medicine, and clinical research, to survivorship and advocacy, the AACR Annual Meeting showcases the outstanding achievements of cancer research institutions worldwide.

CD19 CAR-T cell therapy has significantly improved the prognosis of patients with relapsed/refractory large B-cell lymphoma (r/r LBCL), including diffuse large B-cell lymphoma (DLBCL), primary mediastinal B-cell lymphoma (PMBCL), and LBCL transformed from follicular lymphoma (t-FL) or non-follicular lymphoma (t-NFL).

On July 24, 2020, the U.S. Food and Drug Administration (FDA) granted accelerated approval for Brexucabtagene Autoleucel (Tecartus), a CD19-directed, genetically modified autologous T-cell immunotherapy, for the treatment of adult patients with relapsed or refractory mantle cell lymphoma (MCL).
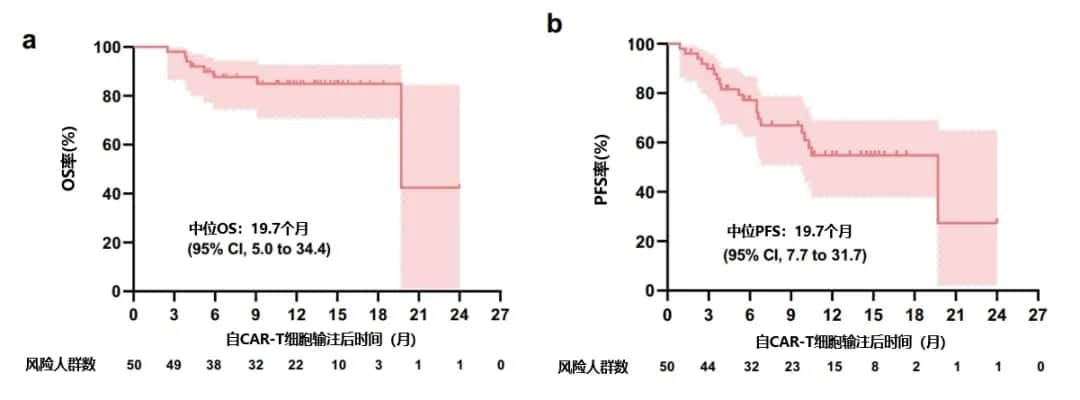
Based on these data, a second-generation bispecific BC19 CAR targeting BCMA and CD19 was designed. This trial reports the preclinical results of BC19 CAR-T cells and the outcomes of R/R MM patients treated in the phase I/II trial.
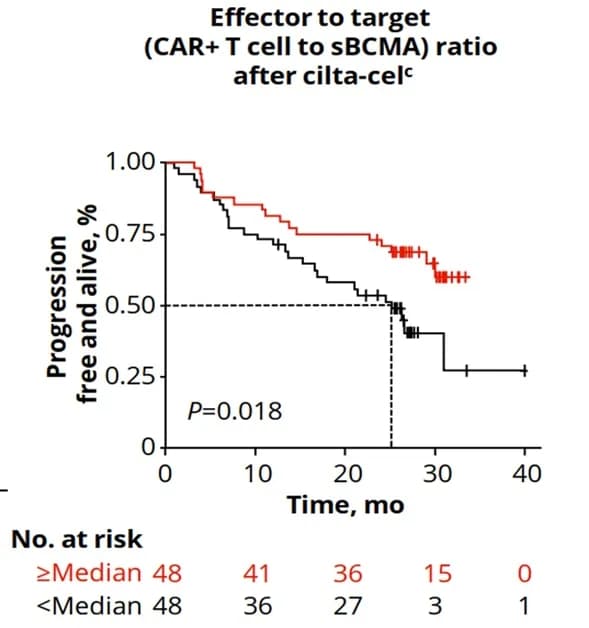
During this EBMT meeting, Professor Qiu Lugui’s team reported the latest subgroup analysis results from the FUMANBA-1 study (Abstract No. OS10-04) on B-cell maturation antigen (BCMA)-targeted chimeric antigen receptor T (CAR-T) cell therapy for relapsed or refractory multiple myeloma (RRMM).


