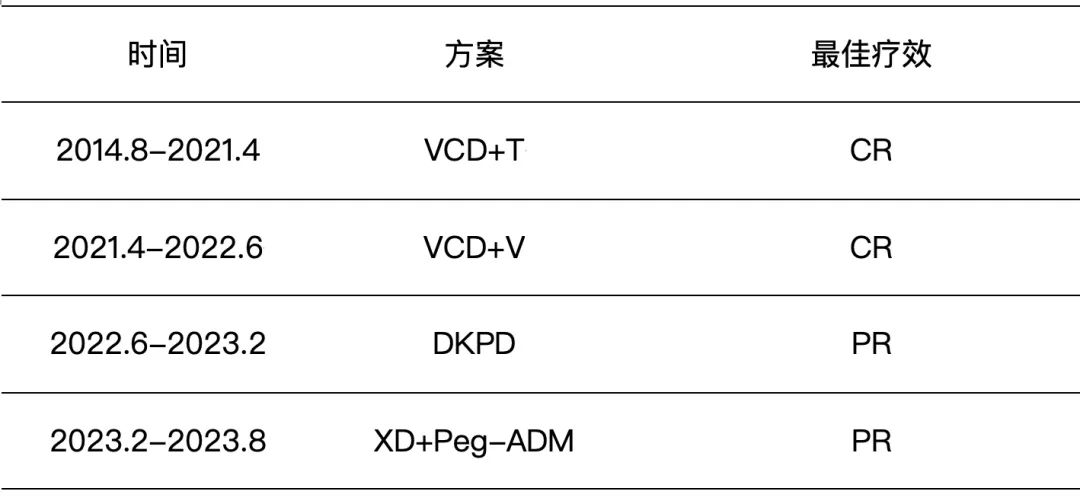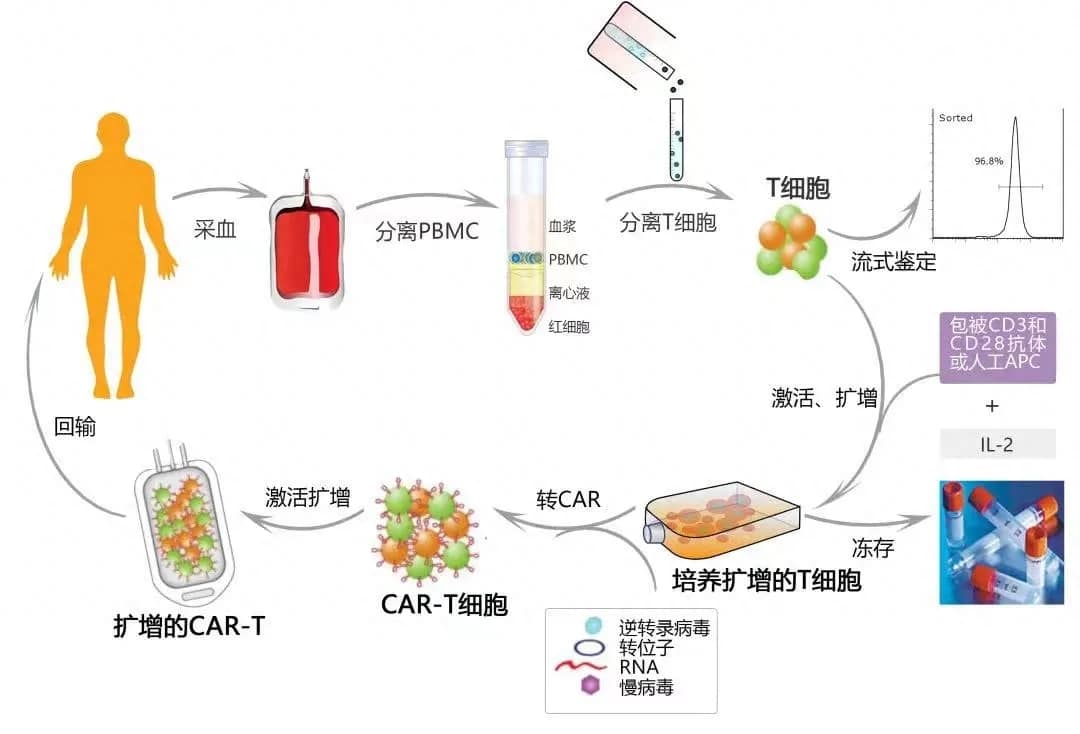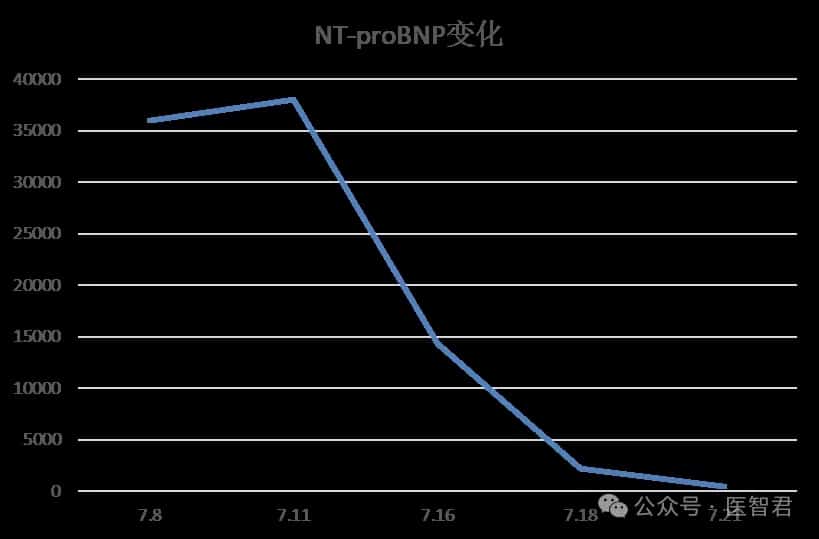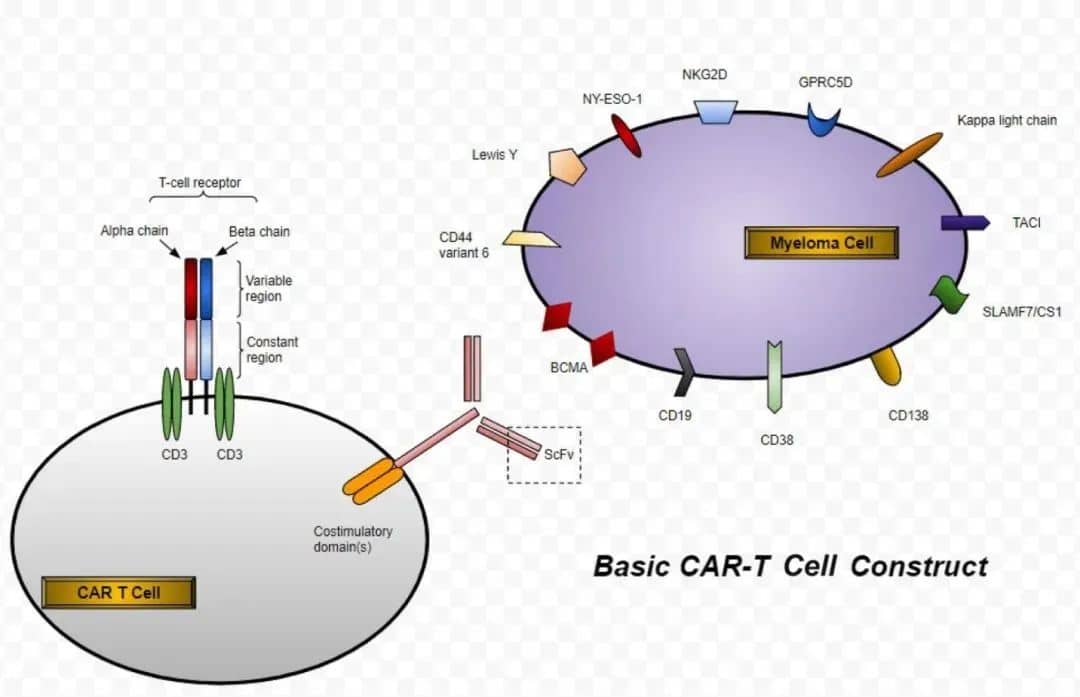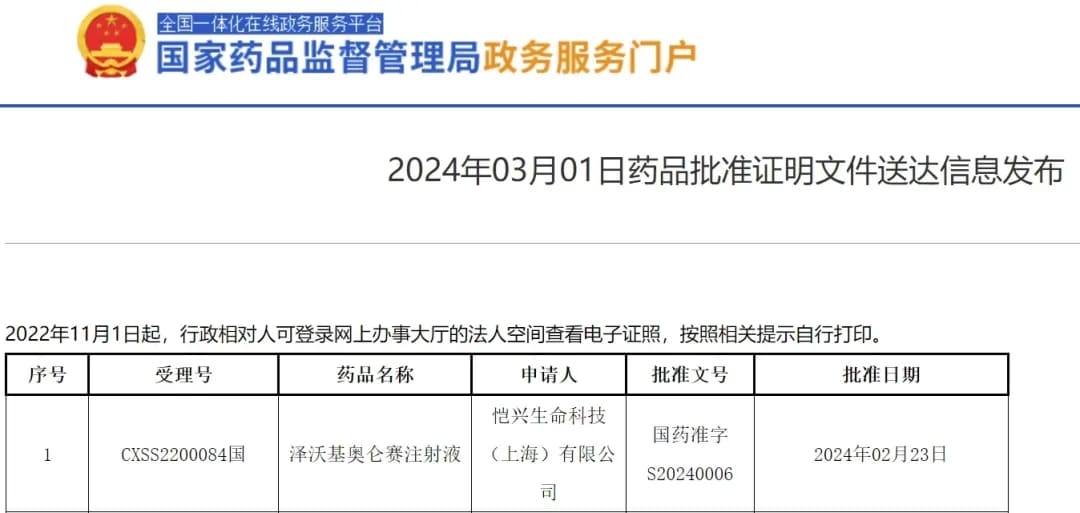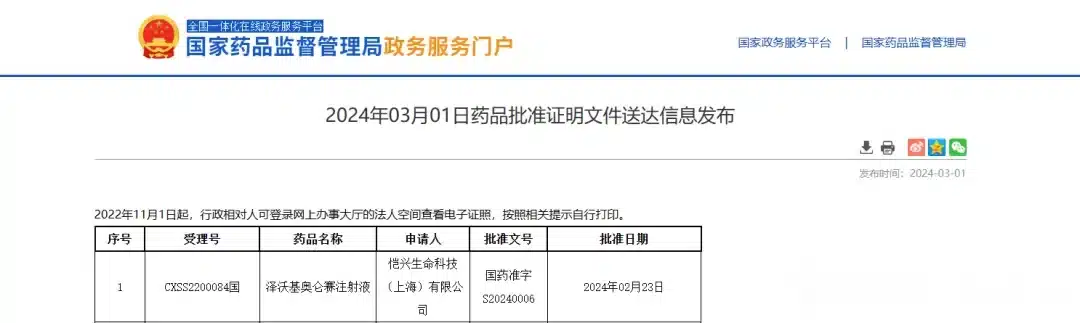
Following the global launch of the first approved TIL cell therapy, lifileucel, the second CAR-T product in China for the treatment of multiple myeloma, Zevorcabtagene Autoleucel (赛恺泽), has finally received approval from the National Medical Products Administration (NMPA) for its new drug application to treat adult patients with relapsed or refractory multiple myeloma.