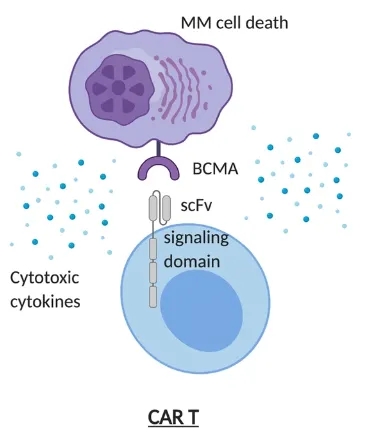
Bluebird bio, a pioneering biotechnology company, in collaboration with Bristol Myers Squibb, has developed a groundbreaking treatment called Abecma, a chimeric antigen receptor (CAR) T-cell therapy that harnesses the power of the body’s immune system to combat this aggressive disease.