Hematological Neoplasms

At the Bone Marrow Transplantation Center of the First Affiliated Hospital of Zhejiang University School of Medicine in China, there is a “Pharmacy God” team that wrestles with the Grim Reaper every day. They use the latest cell therapy technique – Chimeric Antigen Receptor T-cell (CAR-T) therapy to treat malignant hematological diseases, with a success rate of over 90%. Currently in the clinical trial stage, the cost of CAR-T cell preparation is free.

Based on the excellent clinical data, in December 2019, the FDA granted Ciltacabtagene Autoleucel “Breakthrough Therapy Designation”; in February 2020, the European Commission granted it “Orphan Drug Designation”; on June 5, 2020, Legend Biotech officially went public on NASDAQ. In August 2020, Ciltacabtagene Autoleucel also received the first “Breakthrough Therapy” certification from the China National Medical Products Administration (NMPA) Center for Drug Evaluation.
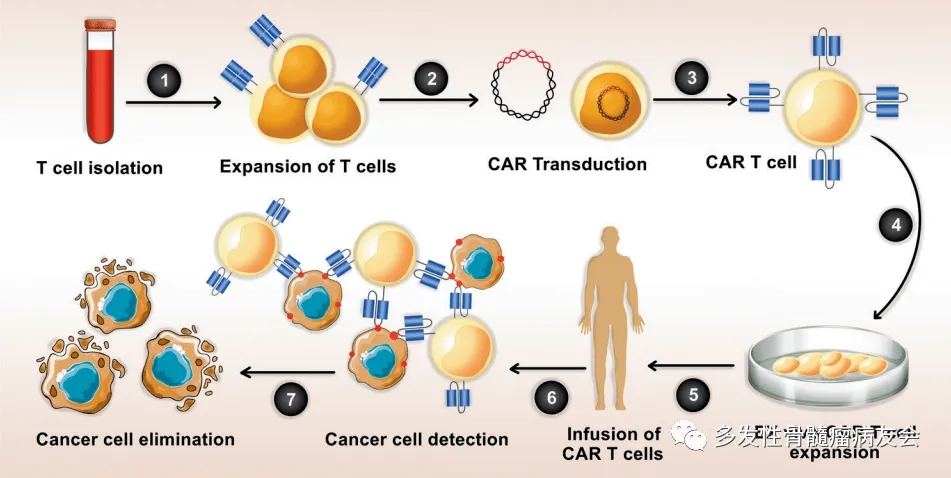
CAR-T cell therapy (Chimeric Antigen Receptor T-cell therapy) is a form of cellular immunotherapy that involves genetically modifying a patient’s T cells to express a specific chimeric antigen receptor (CAR), inducing an anti-tumor response.
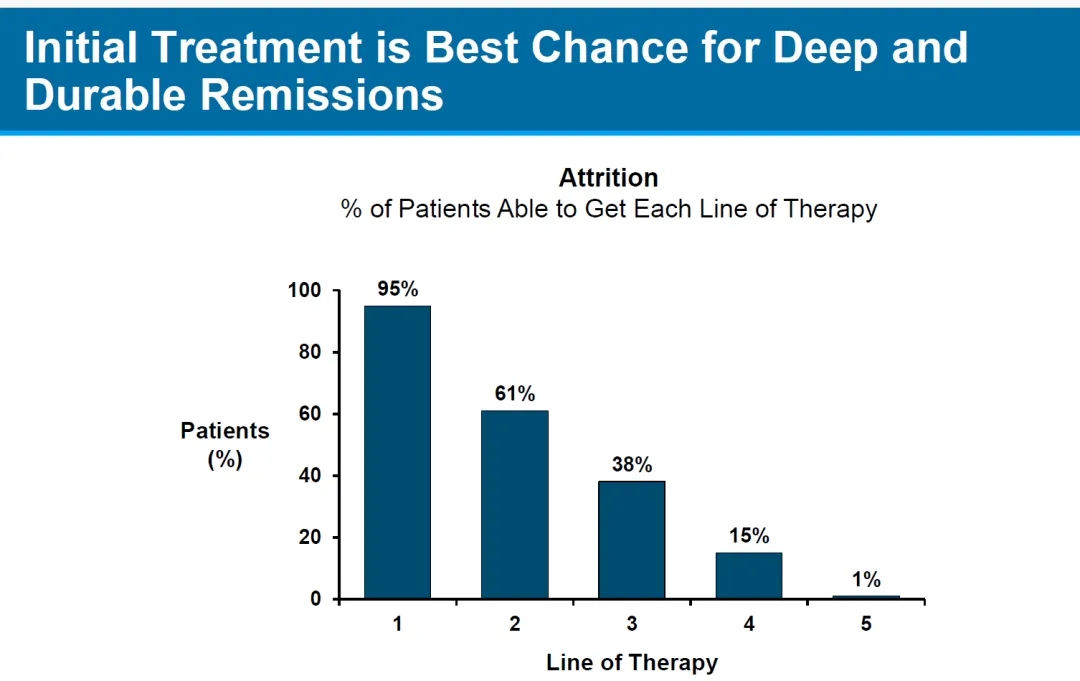
March 15,2024,the FDA Oncologic Drugs Advisory Committee (ODAC) held a full-day meeting to review the benefit/risk profiles of Abecma from BMS/Bluebird Bio and Carvykti from Johnson & Johnson/Legend Biotech, two BCMA CAR-T therapies for frontline treatment.

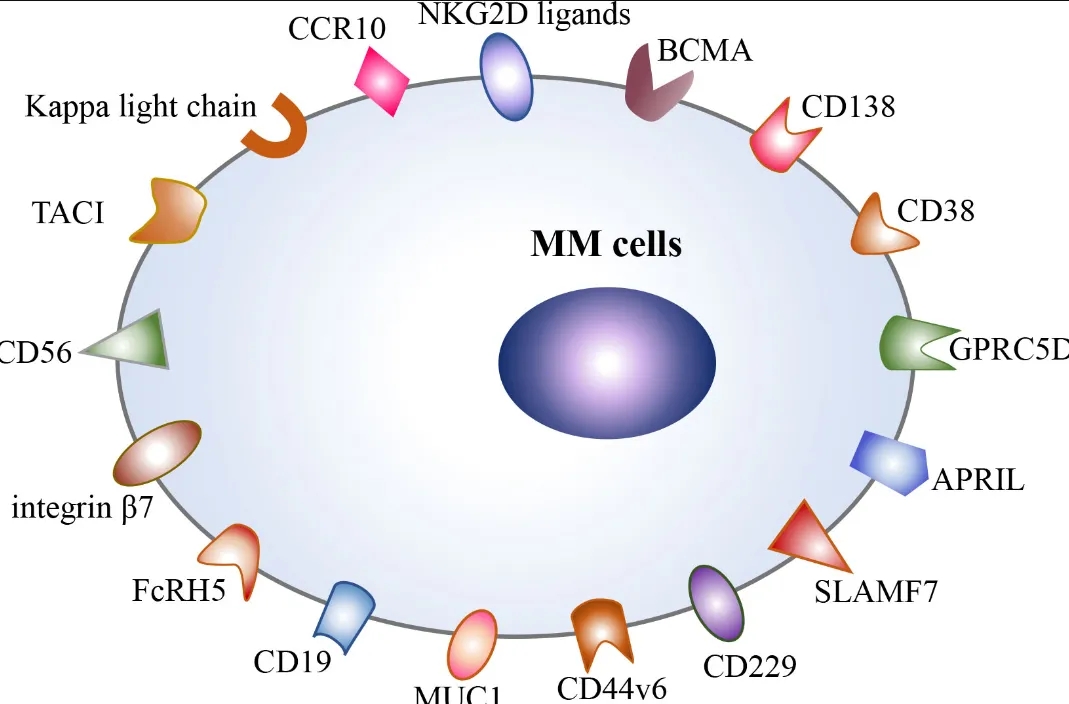
🩸 Breakthrough in Multiple Myeloma Treatment! 🩺 🔬Targeting GPRC5D with CAR-T Cells shows immense potential in treating relapsed or refractory multiple myeloma patients. Multiple myeloma, a malignant disease characterized by clonal plasma cell proliferation in the bone marrow, has remained incurable despite substantial progress in treatment methods such as systemic chemotherapy, radiation therapy, and hematopoietic Read More
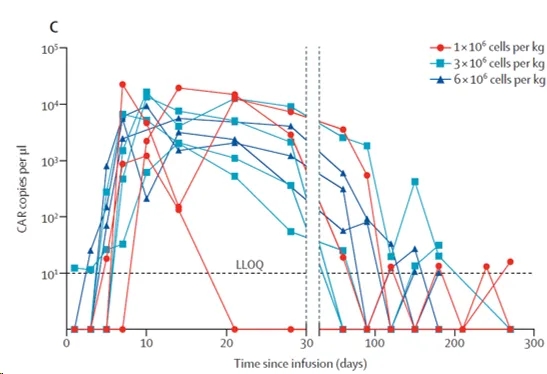
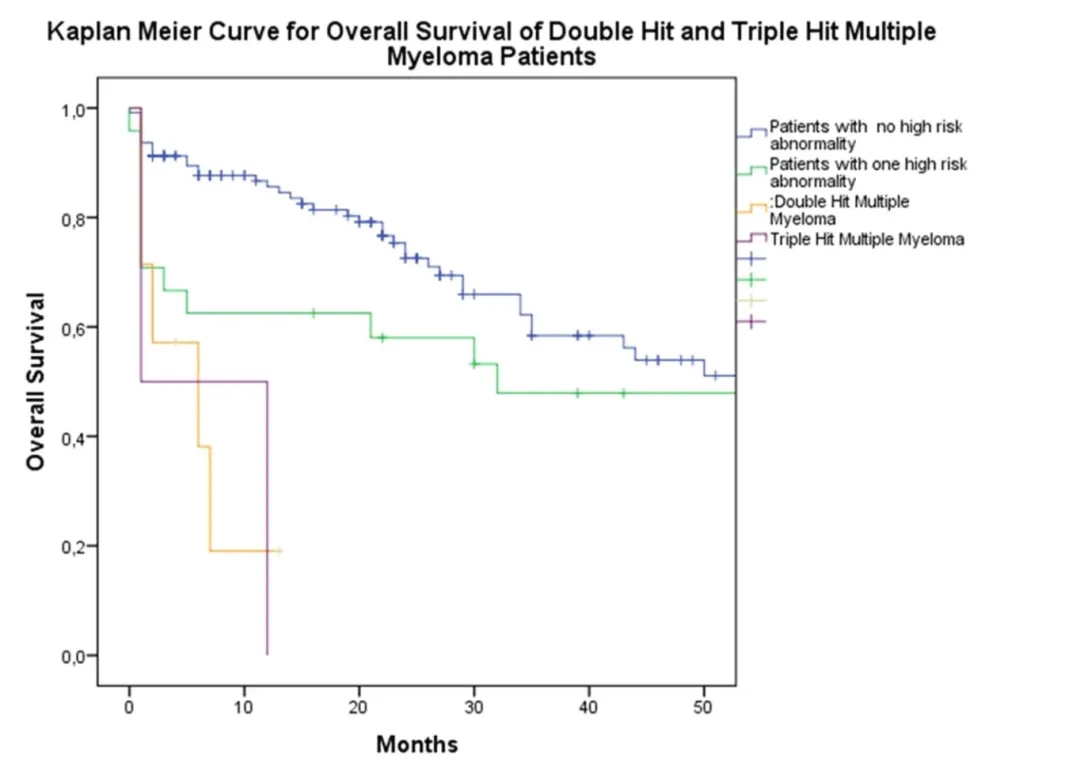
Chimeric antigen receptor (CAR) T-cell therapy targeting B-cell maturation antigen (BCMA) has shown activity in treating relapsed or refractory multiple myeloma