
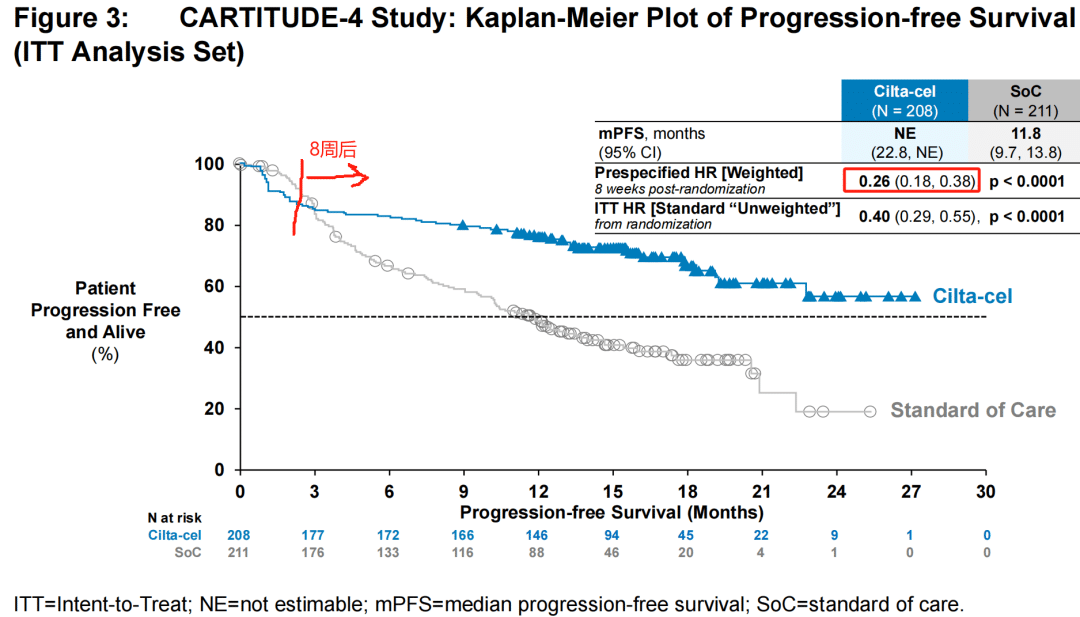
Based on the results of the Phase 3 CARTITUDE-4 study, the U.S. Food and Drug Administration’s (FDA) Oncologic Drugs Advisory Committee (ODAC) voted 11-0 in favor of a positive risk-benefit assessment for Carvykti®.

Based on the results of the Phase 3 CARTITUDE-4 study, the U.S. Food and Drug Administration’s (FDA) Oncologic Drugs Advisory Committee (ODAC) voted 11-0 in favor of a positive risk-benefit assessment for Carvykti®.
At 8:30 pm Beijing time on March 15, 2024, the FDA’s Oncologic Drugs Advisory Committee (ODAC) officially convened, with the meeting lasting over 9 hours.
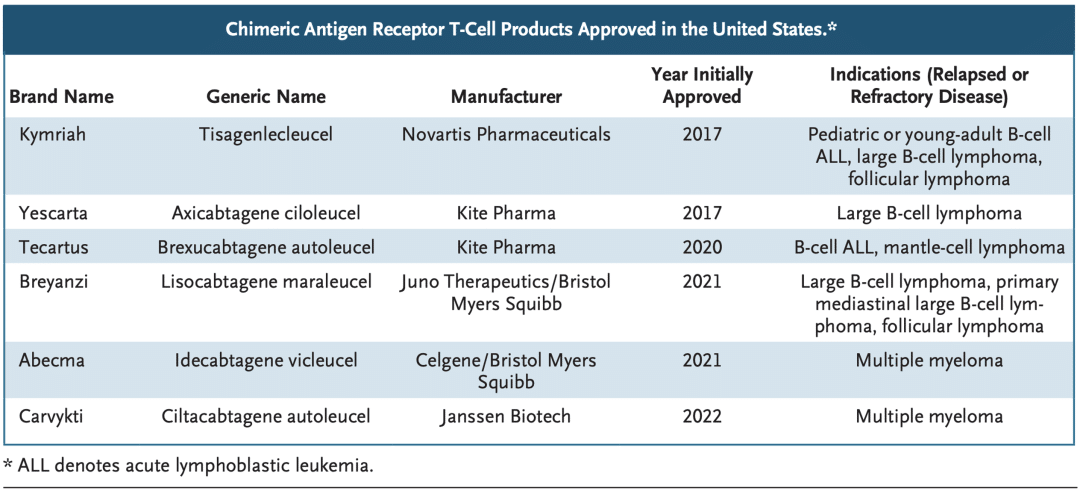
Last week, the FDA released briefing documents from the Oncologic Drugs Advisory Committee (ODAC) meeting regarding Johnson & Johnson’s Carvykti (Ciltacabtagene autoleucel) and Bristol-Myers Squibb’s Abecma (Idecabtagene vicleucel), two CAR-T therapies. The documents highlighted the FDA’s concern over the higher early mortality rates observed in the treatment arms compared to the control arms in the clinical trials for these two CAR-T therapies.

🌎Another BCMA CAR-T therapy for multiple myeloma hits the market in China! – 🔥Long-term efficacy 🔥 🌙China has made significant breakthroughs in the treatment of multiple myeloma with BCMA CAR-T therapy, attracting global attention. Recently, two fully human BCMA CAR-T therapies, Equecabtagene Autoleucel and Zevorcabtagene Autoleucel, have been approved for the treatment Read More

Another BCMA CAR-T therapy for multiple myeloma hits the market in China! – Short-term efficacy China CAR-T therapy In recent years, CAR-T cell therapy has achieved tremendous success in treating hematologic malignancies. Over the past 11 years, China has seen a surge in clinical trials evaluating the safety and efficacy of CAR-T therapy. Read More
On March 16, 2024, Legend Biotech announced that the U.S. FDA Oncologic Drugs Advisory Committee (ODAC) unanimously recommended, with a vote of 11:0, the approval of CARVYKTI® (Ciltacabtagene Autoleucel, cilta-cel) for the treatment of adult patients with relapsed or refractory multiple myeloma who have received at least one prior line of therapy (including a proteasome inhibitor and an immunomodulatory agent) and are refractory to lenalidomide.

Cross-Border Medical Innovation: Singaporean Patient Confronts Multiple Myeloma in China – A Decisive Journey with CAR-T Therapy Singaporean patient, Teresa has been battling multiple myeloma for nearly three years. Despite undergoing various conventional treatments including multi-drug therapy, radiation, and chemotherapy, her condition relapsed with extramedullary plasmacytomas affecting her brain, liver, and multiple bones. Read More

跨境医疗创新:新加坡患者在中国迎战骨髓瘤——一次CAR-T治疗的决定性旅程 新加坡患者Teresa女士,经历了近三年与多发性骨髓瘤的斗争,她目前在多线药物治疗、放化疗后仍面临病情复发,髓外浆细胞瘤同时伴有脑、肝、多处骨病变。近期,她在接受上海嘉会国际医院的Dr. Vicky Lee及其团队线上会诊后,做出了一个重要决定:到中国接受FUCASO CAR-T治疗。 中国不仅在CAR-T治疗领域具有全球领先的临床经验,还有处于全球价格洼地的优质商业化CAR-T产品。目前这些已在中国上市的CAR-T产品产能充足,这对于与死神竞速的患者来说至关重要。同时因其仅一针输注的便捷性,已经备受国际患者的关注。 在上海嘉会国际医院,经验丰富的血液肿瘤专家团队将为Teresa制定个性化的治疗方案,并提供全程、精细化的诊疗服务。作为CAR-T治疗的先行者,嘉会医院即将为Teresa女士带来新生的希望。 我们将持续关注患者的治疗后续,并跟进报道。 #CART #CARTTherapy #HopeReborn #FUCASOApproval #Equecel #MultipleMyeloma #JiahuiHospital #Shanghai #Immunotherapy #MedicalInnovation #MedicalBreakthrough #CancerTreatment #FullyHumanCART #与癌症抗争 #CART治疗之旅 #携手嘉会 #全人源CAR-T #健康重生 #共同祈愿 #骨髓瘤 #多发性骨髓瘤 #Multiplemyeloma #患者故事 #HopeForPatients
On February 28, 2022, Legend Biotech Corporation (LEGN) announced that the U.S. Food and Drug Administration (FDA) has approved its first product, CARVYKTI™ (ciltacabtagene autoleucel; cilta-cel), for the treatment of adult patients with relapsed or refractory multiple myeloma (RRMM) after four or more prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody.

🍒🍎Journey of an Indian Cancer Patient to China: Bringing Gratitude Back to India!🍒🍎 🍅“Before coming to China for treatment, I had many concerns because my condition was very pessimistic at that time. I was worried that I might never see my family again… But since I arrived here, everyone I met has been welcoming. The Read More
By using our site, you agree to our Terms and Conditions and Privacy Policy.Advanced Medicine In China does not provide medical advice, diagnosis, or treatment. The information provided on this site is designed to support, not replace, the relationship that exists between a patient/site visitor and his/her existing physician.
© Copyright 2023 Advanced Medicine In China. All rights reserved.